Studies on New Schiff Bases of Benzoxazole: Synthesis, Anticonvulsant and Neurotoxicity Evaluation
Mohammad Sarafroz1*, Erum Hassan Alameer1, Khadijah Ahmed Alturaiki1, Amina Lutfi Alkhalifah1, Mohd Amir2 and Niyaz Ahmad3
1Department of Pharmaceutical Chemistry, College of Clinical Pharmacy, Imam Abdulrahman Bin Faisal University, P.O. Box 1982, Dammam 3 l44l, Saudi Arabia
2Department of Natural Product and Alternative Medicines, College of Clinical Pharmacy, Imam Abdulrahman Bin Faisal University, P.O. Box 1982, Dammam 3 l44l, Saudi Arabia
3Department of Pharmaceutics, College of Clinical Pharmacy, Imam Abdulrahman Bin Faisal University, P.O. Box 1982, Dammam 3 l44l, Saudi Arabia
Corresponding Author E-mail : drsarafroz@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/360410
Article Received on : 12 June 2020
Article Accepted on : 05 July 2020
Article Published : 07 Aug 2020
Twelve Schiff bases of benzoxazole were prepared by usage of methyl-3-amino-4-hydroxybenzoate. The chemistry of the prepared molecules was established based on the spectral data and tested for anticonvulsant activity using maximal electroshock (MES) induced seizure and subcutaneous pentylenetetrazole (scPTZ). In addition, a rotarod method to detect minimal neurological impairment in mice. In anti-MES test molecules 3d, 3e, 3i, 3j and 3k presented effective action corresponding to hydrophobicity. Other compounds of the series like 3b, 3c, 3g and 3l were remarkably less lipophilic and have some potencies. Compounds 3d and 3j effectively passed the rotarod trial without any mark of CNS deficit. In conclusion, the synthesized compounds with distal aryl groups showed higher hydrophobicity and resulted in better pharmacological action, which can be the future of new promising anticonvulsant drugs.
KEYWORDS:Anticonvulsant; Benzoxazole; Neurotoxicity; Schiff Base
Download this article as:| Copy the following to cite this article: Sarafroz M, Alameer E. H, Alturaiki K. A, Alkhalifah A. L, Amir M, Ahmad N. Studies on New Schiff Bases of Benzoxazole: Synthesis, Anticonvulsant and Neurotoxicity Evaluation . Orient J Chem 2020;36(4). |
| Copy the following to cite this URL: Sarafroz M, Alameer E. H, Alturaiki K. A, Alkhalifah A. L, Amir M, Ahmad N. Studies on New Schiff Bases of Benzoxazole: Synthesis, Anticonvulsant and Neurotoxicity Evaluation . Orient J Chem 2020;36(4). Available from: https://bit.ly/3kjRKt9 |
Introduction
Epilepsy is one of the most undefined diseases of the human CNS that can affect all age groups. Despite ongoing research and studies to explain the exact underlying mechanism of epilepsy and the time and effort required to discover new antiepileptic drugs, the results of epilepsy remission are still insufficient.1-2 Current therapeutic regimens like phenytoin, valproate and carbamazepine have been valued in treating 50% of partial seizures and 60-70% of generalized seizures.3 However, a large number of individuals with epilepsy are insensitive to current medications, and they have been experiencing a wide range of side effects.4-5 Although new medications like pregabalin and retigabine have become available in the last decade, their efficacy remains questionable, and have only been used in some certain types of epilepsy.6-8 Existing AEDs are systemic in their effect, which means they lack selectivity to one target site and as a result it causes a lot of adverse effects such as psychosis, cerebellar syndrome, gingival hyperplasia and cardiac arrhythmia.9-10 It should be noted that the response of treatment is variable among patients due to genetic variation.11
The prevalence of hydrazones among mammalian-derived drugs and biochemical agents can lead to the enormous assumption that such moiety is necessary in pharmacophores.12-13 The recent statistics shown that hydrazones are the new, promising molecules for clinical therapy of epilepsy.14-17 Not only do hydrazones have anticonvulsant potency, but have reported anti-inflammatory and analgesic,18 antimicrobial19 and antitubercular.20-21 In this study, novel hydrazone compounds with pharmacophore will be tested by the standard protocols at different dosage and see whether they reflect high efficacy and even more acceptable health complications. The results exposed that all candidates had more advantages in comparison to older generations chemical entities in both protection and fewer side effects.22-24 For the compound to have anticonvulsant activity a specific sequence of entities is required.25-26 A hydrophobic aryl moiety (A), one or two electron donor atoms (D), a hydrogen bonding domain (HBD) are the key parts concerned for antiepileptic action (Figure 1). The main objective of this study is to synthesize Schiff base ligands and to contribute anticonvulsant activity with efficacy and safety. All synthesized compounds have the above-mentioned moieties like a proximal hydrophobic aryl (A), nitrogen atom as an electron donor (D), -NHC=O as hydrogen bonding domain (HBD) and distal hydrophobic benzoxazole having aryl ring (C) (Figure 2).
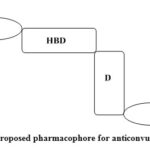 |
Figure 1: Proposed pharmacophore for anticonvulsant action. |
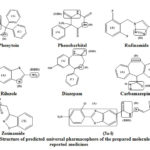 |
Figure 2: Structure of predicted universal pharmacophore of the prepared molecules and reported medicines |
Materials and Methods
Chemistry
The Perkin-Elmer model 240 analyzer was used for elemental analysis and all investigation were reliable within ± 0.4% of the theoretical values. The FT-IR spectra of the compounds were obtained in KBr by FT-IR spectrophotometer (BIO-RAD FTS). Bruker 300MHz and 400 Ultra ShieldTM instruments using DMSO/d6 with TMS as fundamental reference were used to record 1H-NMR spectrum. Mass spectra of the molecules were noted on UPLC-MS/MS (WATERS, MassLynx version 4.1) spectrometer. The melting points recorded on digital melting point apparatus which are unchecked. TLC plates (Merk) was used to determine the completion of the reaction in different solvent systems of benzene: ethanol (2:0.5), toluene: ethylacetate: formic acid (5:4:1), benzene: acetone (8:2). Visualization was made with ultraviolet light. All chemicals and solvents (Spectrochem and CDH) were purified before using them. The spectral records of the new molecules are presented in experimental protocols.
Synthesis
Synthesis of methyl 2-(2-chloro-4-nitrophenyl)-1,3-benzoxazole-5-carboxylate (1)
A mixture of 2-chloro-4-nitrobenzoic acid and methyl-3-amino-4-hydroxybenzoate (0.01mol) refluxed about 15 hrs. Then reaction mixtures poured, cooled over the squashed ice by mixing for acquire the compounds.8,27-28
Synthesis of 2-(2-chloro-4-nitrophenyl)-1,3-benzoxazole-5-carbohydrazide (2)
In absolute ethanol, an equimolar quantity of a mixture of hydrazine hydrate and methyl 2-(2-chloro-4-nitrophenyl)-1,3-benzoxazole-5-carboxylate was heated under the reflux (18-20 hrs). Obtained complex were cooled and the found solids was filtered crystallized by using ethanol.8,27-28
Synthesis of benzoxazole hydrazone-hydrazides (3a-l)
The compound (2) and the un/substituted aromatic aldehydes in an equal mole was refluxed for 12-14 hrs. in the ethanolic medium with little amount of glacial acetic acid as a reagent. Now cooled the mixture and transferred into a snow bath, solid formed was filtered and recrystallized in alcoholic condition to get compound (3a). The other molecules (3b-l) were organized in the same way.18-19
The preparation of the molecules is accessible in scheme 1.
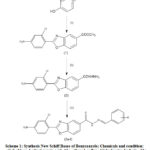 |
Scheme 1: Synthesis New Schiff Bases of Benzoxazole; Chemicals and condition |
2-(2-Chloro-4-nitrophenyl)-N’-[(E)-phenylmethylidene]-1,3-benzoxazole-5-carbo hydrazide (3a)
IR (KBr, cm-1): 3345 (NH), 3001 (CH str), 1701 (C=O), 1600 (C=N), 1300 (NO2). 1H-NMR (DMSO-d6) δ (ppm): 10.78 (s, 1H, NH), 8.49 (s, 1H, CH=), 7.39-8.23 (m, 11H-Ar). Mass m/z: 421.06 (M+1). Anal Calcd for C21H13ClN4O4: C, 60.11; H, 3.19; N, 13.44; Found: C, 59.94; H, 3.11; N, 13.31.
2-(2-Chloro-4-nitrophenyl)-N’-[(E)-(2-chlorophenyl) methylidene]-1,3-benzoxazole-5-carbo hydrazide (3b)
IR (KBr, cm-1): 3311 (NH), 3051 (CH str), 1664 (C=O), 1590 (C=N), 1354 (NO2), 701 (C-Cl). 1H-NMR (DMSO-d6) δ (ppm): 11.04 (s, 1H, NH), 8.90 (s, 1H, CH=), 7.38-8.34 (m, 10H-Ar). Mass m/z: 455.03 (M+1). Anal Calcd for C21H12Cl2N4O4: C, 55.11; H, 2.65; N, 12.21; Found: C, 55.40; H, 2.66; N, 12.31.
2-(2-Chloro-4-nitrophenyl)-N’-[(E)-(3-chlorophenyl) methylidene]-1,3-benzoxazole-5-carbo hydrazide (3c)
IR (KBr, cm-1): 3300 (NH), 3045 (CH str), 1712 (C=O), 1603 (C=N), 1378 (NO2), 704 (C-Cl). 1H-NMR (DMSO-d6) δ (ppm): 10.98 (s, 1H, NH), 9.02 (s, 1H, CH=), 7.33-8.51 (m, 10H-Ar). Mass m/z: 455.03 (M+1). Anal Calcd for C21H12Cl2N4O4: C, 55.11; H, 2.65; N, 12.21; Found: C, 55.40; H, 2.66; N, 12.31.
2-(2-Chloro-4-nitrophenyl)-N’-[(E)-(4-chlorophenyl) methylidene]-1,3-benzoxazole-5-carbo hydrazide (3d)
IR (KBr, cm-1): 3300 (NH), 3045 (CH str), 1712 (C=O), 1603 (C=N), 1409 (NO2), 704 (C-Cl). 1H-NMR (DMSO-d6) δ (ppm): 10.98 (s, 1H, NH), 9.02 (s, 1H, CH=), 7.33-8.51 (m, 10H-Ar). Mass m/z: 455.03 (M+1). Anal Calcd for C21H12Cl2N4O4: C, 55.11; H, 2.65; N, 12.21; Found: C, 55.40; H, 2.66; N, 12.31.
2-(2-Chloro-4-nitrophenyl)-N’-[(E)-(2,6-dichlorophenyl) methylidene]-1,3-benzoxazole-5-carbohydrazide (3e)
IR (KBr, cm-1): 3272 (NH), 3074 (CH str), 1644 (C=O), 1588 (C=N), 1444 (NO2), 691 (C-Cl). 1H-NMR (DMSO-d6) δ (ppm): 10.41 (s, 1H, NH), 8.59 (s, 1H, CH=), 7.34-8.15 (m, 9H-Ar). Mass m/z: 490.19 (M+1). Anal Calcd for C21H11Cl3N4O4: C, 51.12; H, 2.14; N, 11.09; Found: C, 51.51; H, 2.26; N, 11.44.
N’-[(E)-(2-Bromophenyl) methylidene]-2-(2-chloro-4-nitrophenyl)-1,3-benzoxazole-5-carbo hydrazide (3f)
IR (KBr, cm-1): 3378 (NH), 3035 (CH str), 1680 (C=O), 1609 (C=N), 1398 (NO2), 601 (C-Br). 1H-NMR (DMSO-d6) δ (ppm): 10.75 (s, 1H, NH), 8.52 (s, 1H, CH=), 7.29-8.25 (m, 10H-Ar). Anal Calcd for C21H12BrClN4O4: C, 50.15; H, 2.48; N, 11.34; Found: C, 50.48; H, 2.42; N, 11.21.
N’-[(E)-(4-Bromophenyl) methylidene]-2-(2-chloro-4-nitrophenyl)-1,3-benzoxazole-5-carbohydrazide (3g)
IR (KBr, cm-1): 3394 (NH), 3098 (CH str), 1714 (C=O), 1600 (C=N), 1441 (NO2), 619 (C-Br). 1H-NMR (DMSO-d6) δ (ppm): 10.75 (s, 1H, NH), 8.52 (s, 1H, CH=), 7.29-8.25 (m, 10H-Ar). Anal Calcd for C21H12BrClN4O4: C, 50.15; H, 2.48; N, 11.34; Found: C, 50.48; H, 2.42; N, 11.21.
2-(2-Chloro-4-nitrophenyl)-N’-[(E)-(3,5-dibromo-4-hydroxyphenyl) methylidene]-1,3-benzoxazole-5-carbohydrazide (3h)
IR (KBr, cm-1): 3311 (NH), 3064 (CH str), 1711 (C=O), 1609 (C=N), 1397 (NO2), 611 (C-Br). 1H-NMR (DMSO-d6) δ (ppm): 10.88 (s, 1H, NH), 9.51 (s, 1H, OH), 8.71 (s, 1H, CH=), 7.65-8.51 (m, 8H-Ar). Mass m/z: 592.88 (M+1). Anal Calcd for C21H11Br2ClN4O5: C, 42.14; H, 2.00; N, 5.62; Found: C, 42.42; H, 1.86; N, 5.96.
N’-[(E)-(3-Bromo-5-chloro-4-hydroxyphenyl) methylidene]-2-(2-chloro-4-nitrophenyl)-1,3-benzoxazole-5-carbohydrazide (3i)
IR (KBr, cm-1): 3289 (NH), 3007 (CH str), 1714 (C=O), 1611 (C=N), 1363 (NO2), 723 (C-Cl), 600 (C-Br). 1H-NMR (DMSO-d6) δ (ppm): 11.01 (s, 1H, NH), 9.58 (s, 1H, OH), 8.62 (s, 1H, CH=), 7.62-8.26 (m, 8H-Ar). Mass m/z: 548.13 (M+1). Anal Calcd for C21H11Br2ClN4O5: C, 46.11; H, 1.91; N, 10.35; Found: C, 45.85; H, 2.02; N, 10.18.
N’-[(E)-(3-Bromo-4-hydroxyphenyl) methylidene]-2-(2-chloro-4-nitrophenyl)-1,3-benzoxazole-5-carbohydrazide (3j)
IR (KBr, cm-1): 3300 (NH), 3014 (CH str), 1716 (C=O), 1568 (C=N), 1404 (NO2), 598 (C-Br). 1H-NMR (DMSO-d6) δ (ppm): 10.89 (s, 1H, NH), 9.47 (s, 1H, OH), 8.41 (s, 1H, CH=), 6.98-8.15 (m, 9H-Ar). Anal Calcd for C21H11Br2ClN4O5: C, 48.78; H, 2.05; N, 10.75; Found: C, 48.91; H, 2.35; N, 10.86.
N’-[(E)-(2-Bromo-5-hydroxyphenyl) methylidene]-2-(2-chloro-4-nitrophenyl)-1,3-benzoxazole-5-carbohydrazide (3k)
IR (KBr, cm-1): 3300 (NH), 3014 (CH str), 1716 (C=O), 1568 (C=N), 1404 (NO2), 598 (C-Br). 1H-NMR (DMSO-d6) δ (ppm): 10.56 (s, 1H, NH), 9.44 (s, 1H, OH), 8.63 (s, 1H, CH=), 6.64-8.09 (m, 9H-Ar). Anal Calcd for C21H11Br2ClN4O5: C, 48.78; H, 2.05; N, 10.75; Found: C, 48.91; H, 2.35; N, 10.86.
2-(2-Chloro-4-nitrophenyl)-N’-[(E)-(3,5-dibromophenyl) methylidene]-1,3-benzoxazole-5-carbohydrazide (3l)
IR (KBr, cm-1): 3361 (NH), 2991 (CH str), 1760 (C=O), 1561 (C=N), 1415 (NO2), 613 (C-Br). 1H-NMR (DMSO-d6) δ (ppm): 11.16 (s, 1H, NH), 8.44 (s, 1H, CH=), 7.61-8.37 (m, 9H-Ar). Anal Calcd for C21H11Br2ClN4O4: C, 43.31; H, 2.11; N, 9.98; Found: C, 43.59; H, 1.92; N, 9.68.
Pharmacological Activity
Anticonvulsant Study
All synthesized compounds were dissolved in PEG and tested on albino mice. Two methods like MES induced seizures and scPTZ were used for the anticonvulsant study of synthesized molecules by following the procedure of the National Institute of Health Anticonvulsant Screening Program.29-30 The anticonvulsant and neurotoxicity results are stated in Table 2.
Maximal Electroshock Induced Seizure Model
Electroconvulsive shock (60 Hz and 50 mA for 0.2 sec) was fetched via corneal probes to persuade maximal seizures in mice, the several stages of spasm are flexion, extension, clonus, and stupor. Subsequently, the electric stimulus occurred, the extent segments were distinguished at 0.5 and 4 hrs after the drug application. Finally, the reduction in the period of the hindlimb extension was measured as protecting action.29,31-33
Subcutaneous Pentylenetetrazole Seizure Test
Inalbino mice, seizures were produced by i.p. injection of subcutaneous pentylenetetrazole (scPTZ) at 75 mg/kg after that test compounds were administered and the animals were observed for half an hour. Inhibition to detect in contrast to the spread of attacks as a minimum of 5 s period was defined as the safety against clonic tremor.31,34
Neurotoxicity Study
The rotarod method is used to identify minimal neurological impairment.35-36 The neurotic management was experienced in mice by the rotarod method. The incapability of a treated mouse to keep a balance for at least 1 min on a 3.2cm diameter slowly revolving rod (6 rpm) was used as the end point signifying motor impairment.
Lipophilicity Determination
The bioactivity of novel medicinal compounds, acting on CNS, were correlated with partition coefficient or hydrophobicity and was determined by the known procedure37. However, if the compound has a log P ≈ 2 it will lead to maximal action. In the present work, we connected the calculated log P value with the potency of the newer synthesized compounds and were calculated by known method i.e. chloroform phosphate buffer technique.38
Result and Discussion
Twelve new hydrazones were synthesized via the condensation of methyl-3-amino-4-hydroxybenzoate with 2-chloro-4-nitrobenzoic acid followed by treatment with hydrazine hydrate, are shown in Scheme 1. Hydrazide of benzoxazole (2) was refluxed for 18-20 hrs with appropriate aromatic aldehydes to form novel hydrazones (3a-l). The completion of the reaction was checked by thin-layer chromatography. All synthetic compounds are novel and their anticonvulsant activities have not been specified in the literature. The physical parameters of the prepared molecules are accessible in Table 1. Structural clarification of the prepared molecules has been recognized on the basis of spectral means. Overall, 1H-NMR spectra of the most analogs displayed characteristic singlet signs for =CH and -NH groups have been detected on δ 8.41–9.02 ppm and δ 10.41–11.16 ppm, respectively. FT-IR spectra of mostly derivatives exposed absorption band at around 3361-3271cm-1 and 3098-2991 confirming the existence of -NH and -CH correspondingly, recognized the synthesized molecules.
Table 1: Physical parameters of the title compounds (3a-l)
|
Code No. |
R |
Mol. formulaa |
M.Pb (°C) |
% Yield |
Log Pc |
Rfd Value |
|
3a |
H |
C21H13ClN4O4 |
210-212 |
54 |
0.57 |
0.71 |
|
3b |
2-Cl |
C21H12Cl2N4O4 |
215-217 |
71 |
1.10 |
0.64 |
|
3c |
3-Cl |
C21H12Cl2N4O4 |
210-212 |
45 |
1.33 |
0.47 |
|
3d |
4-Cl |
C21H12Cl2N4O4 |
220-222 |
75 |
2.15 |
0.88 |
|
3e |
2,6-dichloro |
C21H11Cl3N4O4 |
230-232 |
74 |
|
|
|
3f |
2-Br |
C21H12BrClN4O4 |
200-202 |
71 |
2.46 |
0.74 |
|
3g |
4-Br |
C21H12BrClN4O4 |
205-207 |
49 |
1.21 |
0.72 |
|
3h |
3,5-dibromo-4-hydroxy |
C21H11Br2ClN4O5 |
240-242 |
64 |
0.60 |
0.79 |
|
3i |
3-bromo-5-chloro-4-hydroxy |
C21H11BrCl2N4O5 |
230-232 |
59 |
1.94 |
0.77 |
|
3j |
3-bromo-4-hydroxy |
C21H12BrClN4O5 |
190-192 |
40 |
2.04 |
0.55 |
|
3k |
2-bromo-5-hydroxy |
C21H12BrClN4O5 |
200-202 |
49 |
2.11 |
0.75 |
|
3l |
3,5-dibromo |
C21H11Br2ClN4O4 |
225-227 |
78 |
1.14 |
0.71 |
aSolvent of crystallization — ethanol.
bMelting point of the compounds at their decay.
cLog P was deliberate by absorbance records, chloroform/phosphate buffer at 28 °C.
dSolvent system — benzene:acetone (8:2), benzene: ethanol (2:0.5), toluene:ethylacetate: formic acid (5:4:1).
Anticonvulsant actions of the compounds were accomplished via MES and scPTZ procedure and neurological deficit were determined by rotarod trials29,31-33,35-36. All synthesized compounds were liquified in polyethylene glycol and tested on albino mice (25-30 g), and are specified in Table 2. The current clinically used AEDs vary in their management of epilepsy. For comparison, phenytoin and carbamazepine were used as standards. All the new chemical entities were active in the MES test except 3a and 3h, revealing their capacity to avoid seizure spared. At a dose of 100 mg/kg, compounds 3d, 3e, 3i, 3j and 3k exhibited protection in half or more of the tested mice. Compounds 3b, 3c, 3f and 3l showed safety at higher dosage after 0.5 hr but did not display protection after 4 hrs time intervals. Further, compounds 3d, 3e, 3g, 3i, 3j and 3k showed protection at both time periods. In this way, the most compounds showed anticonvulsant actions at 0.5 hr, signifying that they have quick action and shorter period of action.
Table 2: Anticonvulsant and neurotoxicity study of the title compounds (3a-l)
|
Code No. |
i.p. administration in micea |
Neurotoxicity screena |
||||
|
MES screen |
scPTZ screen |
|||||
|
0.5h |
4h |
0.5h |
4h |
0.5h |
4h |
|
|
3a |
‒ |
‒ |
‒ |
‒ |
× |
× |
|
3b |
300 |
‒ |
‒ |
‒ |
300 |
‒ |
|
3c |
300 |
‒ |
‒ |
‒ |
300 |
‒ |
|
3d |
100 |
300 |
300 |
‒ |
‒ |
‒ |
|
3e |
100 |
300 |
‒ |
‒ |
‒ |
300 |
|
3f |
300 |
‒ |
300 |
‒ |
300 |
‒ |
|
3g |
300 |
300 |
‒ |
‒ |
300 |
× |
|
3h |
‒ |
‒ |
‒ |
‒ |
× |
× |
|
3i |
100 |
300 |
300 |
‒ |
300 |
‒ |
|
3j |
100 |
300 |
300 |
‒ |
‒ |
‒ |
|
3k |
100 |
300 |
300 |
‒ |
300 |
‒ |
|
3l |
300 |
‒ |
‒ |
‒ |
300 |
300 |
|
Phenytoinb |
30 |
30 |
‒ |
‒ |
100 |
100 |
|
Carbamazepineb |
30 |
100 |
100 |
300 |
300 |
300 |
aTest compounds were injected to mice (30, 100 and 300 mg/kg). The data in the table show the lowest dose whereby effects were recognized in half or more of the mice. The mice were detected 0.5 and 4 hrs after the i.p. administration of test molecules. The dash (‒) indicates absence of result at maximum dosage (300 mg/kg) and cross (×) indicates not tested. Polyethylene glycol (0.1 ml, i.p.) was used as control solvent.
bStatistics from reference.8,14,21
In scPTZ, five molecules 3d, 3f, 3i, 3j, and 3k revealed effects at 0.5 h time interval at a dose of 300 mg/kg, viewing that rapid onset and but small action. In neurotoxicity study, all the candidates showed neurotoxic effects except 3d and 3j (300 mg/kg). Only one compound 3e showed late toxicity after 4 hrs as compared to reference drug carbamazepine. Compound 3l exposed toxicity at both periods, on the other hand, all the compounds were less neurogenic than phenytoin.
Compounds 3d, 3e 3i, 3j and 3k were observed to have higher lipophilicity having influential anticonvulsant action. Other compounds of the series like 3b, 3c 3g and 3l were remarkably less lipophilic and had some potency.
Conclusion
In the current study, twelve Schiff base ligands were synthesized and exposed for antiepileptic study via MES and scPTZ methods. All compounds showed good to moderate effects except 3a and 3h. Preliminary study shows that the compounds 3d, 3e, 3i, 3j and 3k were exhibited potent actions at a dose of 100 mg/kg at 0.5 hr, it may be valued as prototypic contenders. The data of anticonvulsant studies showed that each molecule displayed characteristics decline of hindlimb stimulant extensor period. Furthermore, antiepileptic studies of different mixes were seen as many less dynamic than reference drugs phenytoin and carbamazepine. Subsequently, it has been observed that the result is due to the presence of auspicious key moieties like distal aryl ring keeping hydrophobic character, hydrogen bonding domain -NHC=O group, electron donor =N‒ atom, electron-withdrawing group and extra aryl group as a hydrophobic site. According to the results, it indicates that the presence of halo substitution on the distal aryl ring increases the van der Waals interaction at the target site and similarly rises hydrophobic character as well as potency. The distal aryl ring (benzylidene) is important for the pharmacokinetic assets of the molecules because the change in the substitution at the benzylidene ring was found to mark pharmacological effects. Compounds 3d, 3e 3i, 3j and 3k were detected with higher lipophilicity having significant anticonvulsant action. Other compounds like 3b, 3c, 3g and 3l were remarkably less lipophilic and had some potency. In conclusion, Schiff bases were found to have anticonvulsant effects and prompt a promising molecule by striking pharmacological decency.
Acknowledgement
Authors are very thankful to College of Clinical Pharmacy, Imam Abdulrahman Bin Faisal University, Dammam, Kingdom of Saudi Arabia for synthesis and spectral characterization. We are also appreciations to the Antiepileptic Drug Development Program for finalizing the assessment.
Conflict of Interest
The authors declare no conflict of interest.
References
- Pottoo, F. H.; Tabassum, N.; Javed, M. N.; Nigar, S.; Rasheed, R.; Khan, A.; Barkat, M. A.; Alam, M. S.; Maqbool, A.; Ansari, M. A.; Barreto, G. E.; Ashraf, G. M. Neu. 2019, 56(2), 1233-1247.
CrossRef - Ahmad, N.; Ahmad, R.; Alrasheed, R. A.; Almatar, H. M. A.; Al-Ramadan, A. S.; Amir, M.; Sarafroz, M. 2020, 12, 1-34.
CrossRef - Nigar, S.; Pottoo, F. H.; Tabassum, N.; Verma, S. K.; Javed, M. N. Adv. Med. Pharm. Sci. 2016, 10, 1-9.
CrossRef - Ahmad, N.; Ahmad, R.; Al Qatifi, S.; Alessa, M.; Al Hajji, H.; Sarafroz, M. BMC Chem. 2020, 14(1), 1-15.
CrossRef - Łuszczki, J. J. Rep. 2009, 61(2), 197-216.
CrossRef - Naveeda, R.; Anum, S.; Saima, K. J. App. Sci. Res. Rev. 2018, 5, 2-10.
- Khatoon, Y.; Singh, V.; Sarafroz, M. J. Pharm. Sci. Rev. 2018, 48(2), 70-78.
- Sarafroz, M.; Khatoon, Y.; Ahmad, N.; Amir, M.; Salahuddin.; Pottoo, F. H. J. Chem. 2019, 35(1), 64-70.
CrossRef - Remi, J.; Huttenbrenner, A.; Feddersen, B.; Noachtar, S. Res. 2010, 88, 145-150.
CrossRef - Sarafroz, M.; Khatoon, Y.; Ahmad, N.; Amir, M.; Salahuddin, Pottoo, F. H.; Taleuzzaman, M.; Ahmad, W. J. Pharm. Sci. Res. 2020, 11(1), 137-45.
- Balestrini, S.; Sisodiya, S. M. Let. 2018, 22, 27-39.
CrossRef - Ali, M. R.; Marella, A.; Alam, T. M.; Naz, R.; Akhter, M.; Shaquiquzzaman, M.; Saha, R.; Tanwar, O.; Alam, M. M.; Hooda, J. J. Pharm. 2012, 23(4), 193- 202.
- Asif, M.; Husain, A. App. Chem. 2013, 66, 1-7.
CrossRef - Agrawal, S.; Jain, J.; Kumar, A.; Gupta, P.; Garg, V. Res Rep Med Chem. 2014, 4, 47-58.
CrossRef - Amir, M.; Ali, I.; Hassan, M. Z.; Mulakayala, N. Der. Pharm. Chem. L. Sci. 2014, 347, 958-968.
CrossRef - Kumar, D.; Sharma, V. K.; Kumar, R.; Singh, T.; Singh, H.; Singh, A. D.; Roy, R. K. J. 2013, 12, 628-640.
- Shaquiquzzaman, M.; Khan, S. A.; Amir, M.; Alam, M. M. Enz. Inh. Med. Chem. 2012, 27, 825-831.
CrossRef - Salgın-Goksen, U.; Gokkhan-Kelekc, N.; Goktas, O.; Koysal, Y.; Kilic, E.; Isik, S.; Aktay, G.; Ozalp, M. Med. Chem. 2007, 15, 5738-5751.
CrossRef - Zhang, J.; Shen, T.; Xu, L.; Shen, F.; Qin, Q.; Ma, C.; Song, Q. Comm. 2010, 40, 814-820.
CrossRef - Therese, S. K.; Malika, G. G. J. Chem. 2017, 33(1), 335-345.
CrossRef - Judge, V.; Narasimhan, B.; Ahuja, M.; Sriram, D.; Yogeeswari, P.; Clercq, E. D.; Pannecouque, C.; Balzarini, J. Chem. Res. 2012a, 21(8), 1935-1952.
CrossRef - Hanaya, R.; Arita, K. Med. Chi. 2016, 56(5), 205-220.
CrossRef - Sridhar, S. K.; Pandeya, S. N.; Stables, J.; Ramesh, A. J. Pharm. Sci, 2002, 16(3), 129-132.
CrossRef - Kubota, M.; Nishi-Nagase, M.; Sakakihara, Y.; Noma, S.; Nakamoto, M.; Kawaguchi, H.; Yanagisawa, M. Deve. 2000, 22(4), 230-233.
CrossRef - Dimmock, J. R.; Vashishtha, S. C.; Stables, J. P. J. Med. Chem. 2000, 35(2), 241-248.
- Dimmock, J. R.; Vashishtha, S. C.; Stables, J. P. Pharm. 2000b, 55, 490-494.
- Siddiqui, N.; Sarafroz, M.; Alam, M. M.; Ahsan, W. Polo. Pharm. Drug Res. 2008, 4(65), 449-455.
- Khatoon, Y.; Shaquiquzzaman, M.; Singh, V.; Sarafroz, M. App. Pharm. Sci. 2017, 7(7), 158-167.
- Krall, R. J.; Penry, J. K.; White, B. G.; Kupferberg, H. J.; Swinyard, E. A. Epi. 1978, 19(4), 409-428.
CrossRef - Porter, R. J.; Cereghino, J. J.; Gladding, G. D.; Hessie, B. J.; Kupferburg, H. J.; Scoville, B. Cli, 1984, 51, 293-305.
CrossRef - Pandeya, S. N.; Ponnilavarasan, I.; Pandey, A.; Lakhan, R.; Stables, J. P. Pharm. 1999, 54(12), 923-925.
- Rollas, S.; Kucukguzel, S. G. Mol. 2007, 12(8), 1910-1939.
CrossRef - Tuncbilek, M.; Altanlar, M. Der. Pharm. 2006, 339(4), 213-216.
CrossRef - Racine, R. J. Clin. Neu. 1972, 32, 281-294.
CrossRef - Dunham, N. W.; Miya, T. S. Am. Pharm. Ass. Sci. 1957, 46, 208-209.
CrossRef - Kucukguzel, I.; Kucukguzel, S. G.; Rollas, S.; Sanis, G. O.; Ozdemir, O.; Bayrak, I.; Altug, T.; Stables, J. P. L. Farma, 2004, 59(11), 893-901.
CrossRef - Lien, E. J.; Liuo, R. C. H.; Shinoucla, H. G. Pharm. Sci. 1979, 68, 463-468.
CrossRef - Farrar, V. A.; Ciechanowicz-Rutkowska, M.; Grochowski, J.; Serda, P.; Pilati, T.; Filippini, G.; Hinko, C. N.; El-Assadi, A.; Moore, J. A.; Edafiogho, I. O.; Andrews, C. W.; Cory, M.; Nicholson, J. M.; Scott, K. R. Med. Chem. 1993, 36, 3517-3525.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









