Structural Characterization of Luteolin Glycosides Flavonoids from Indian Plantation White Sugar
Vikesh Kumar
Department of Chemistry Awadhesh Pratap Singh University, Rewa, Madhya Pradesh- 486003, India
Corresponding Author E-mail : vikeshkumaraps@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/360425
Article Received on : 15 July 2020
Article Accepted on : 16 Aug 2020
Article Published : 29 Jul 2020
Luteolin flavonoid is useful in a variety of dietary constituents and can be used as medicine to protect and suppress the growth of different human cancers. These flavonoids may increase the level of reactive oxygen species, and have value as chemopreventive substances. In this study, a medium grade (L-30) sugar was obtained from a reputed north Indian sugar manufacturer which had turned slightly yellowish due to a long storage period for flavonoids identification. Flavonoids are more stable than other sugar colorants and persist into sugar crystals. A resin-based column chromatography method has been developed for the extraction of three luteolin glycosides flavonoids from Indian plantation white sugar. The fractionation of the colorants was done according to their size by gel permeation chromatography, and finally their isolation and purification by thin-layer chromatography. The detected flavonoids were: luteolin-6-C-β-glucopyranoside, luteolin-7-O-β-glucopyranoside and luteolin-7-O-β-galactopyranoside. Ultraviolet and Nuclear magnetic resonance spectroscopy techniques were used for the structural characterization of flavonoids.
KEYWORDS:Extraction; Flavonoid; Plantation White Sugar; Resin
Download this article as:| Copy the following to cite this article: Kumar V. Structural Characterization of Luteolin Glycosides Flavonoids from Indian Plantation White Sugar. Orient J Chem 2020;36(4). |
| Copy the following to cite this URL: Kumar V. Structural Characterization of Luteolin Glycosides Flavonoids from Indian Plantation White Sugar. Orient J Chem 2020;36(4). Available from: https://bit.ly/30UQcwI |
Introduction
The quality of sugar is directly related to its color value. The color incorporated in sugar crystals may originate from the cane plant itself or may be formed during processing. The former comprises colorants such as chlorophyll, carotenes, xanthophylls, anthocyanins, and flavonoids.1-2 Flavonoids such as tricin, isoorientin, isovitexin, and apigenin glycosides have been identified in sugarcane leaves, liquor, and molasses.3-8 However, flavonoids have promising applications in food and pharmaceuticals.9-10
The luteolin group of flavonoids, sugar are joined to the A ring by carbon-carbon bonds at 6 or 8 positions that occur as C-glycosides. Sugar house products may contain luteolin as it does in the case of other mill syrup and molasses. These phenomena developed from other studies dealing with luteolin-6-C-glucoside, iso-orientin-0-rhamnosylglucoside, luteolin-6-C-glucosyl-7-0-glucoside, iso-orientin-tri-0-glucoside and luteolin-6-8-di-C-glycosides in sugarcane leaf11, and iso-orientin-7-0-methyl ether, iso-orientin7,3’-0-dimethyl ether, 6-methoxyluteolin, orientin-7,3’-0-dimethyl ether and other luteolin derivatives in mill syrup.12-13 Luteolin, a dietary flavonoid is possessed to have anti-thyroid, anti-viral, anticancer, and antioxidant,14-15 characteristics found in fruits, herbs,16 sugarcane mill syrup, and bagasse.17-18 Thus, analysis of flavonoids in sugar and other sugar products commodities like bagasse, mill syrup, and molasses have become essential requirements for the sugar industry.
These flavonoids find their way into the sugar crystal through sugarcane, sugar production process, and enzymatic reaction. No systematic study has been carried out to date to isolate and identify the natural colorants persisting into the granulated direct consumption white sugar made in our country. It is imperative to determine the flavonoids components in sugar samples in India. This paper aims to provide the structural characterization of sugar flavonoids that make it such a useful recipient in the pharmaceutical and sugar industry. The study also includes the application of gel permeation chromatography for the fractionation of sugar flavonoids. Spectral analysis like UV and NMR were used for flavonoids characterization.
Experimental
Chemicals
All solvents like methanol, hydrochloric acid, and ammonia (HPLC grade) were purchased from Merck (Mumbai, India).
Sample Collection
A medium grade (L-30) sugar was obtained in 2019 from a reputed North Indian sugar manufacturer.
Preparation of Solution
Plantation white sugar (25 g) was diluted with 100 ml distilled water to prepare a 25 0Bx (degree Brix) solution. The solution was filtered and the pH was adjusted to about 4 with concentrated HCl.19
Extraction and Cleanup
A XAD-4 macroporous adsorption resin (60 mesh particle size, pore diameter 40 A0, surface area =725 m2/g) was used for recovering sugar flavonoids. The resin was filled in a chromatographic column preconditioned with hydrochloric acid and sodium hydroxide.20 The sugar extract was collected into a 100 ml beaker. Dry colorants (20 g) were dissolved with 100 ml distilled water and 1-2 drops of concentrated HCl was added to precipitate polymeric colorant.19 For flavonoids recovery, a mixture of methanol: ammonia: water (40:4:56) was used. The solution was filtered with Millipore 0.22 μm sterile filter, the solution was then adsorbed onto dextran gel- Sephadex (particle size = 50-150µ, water regain = 5 ml/g, gel bed volume = 10 ml/g) at a rate of 0.33 ml/min and elution was done with water at the same rate. Three fractions were collected separately for each technique which was then chromatographed on cellulose TLC plate.20 The fractions were evaporated and investigated for identification.
Analysis
The final extracts were analyzed in DMSO-d6 containing TMS as the internal standard reference (JEOL AL 500 MHz)1H and13 C NMR spectrometer. Shimadzu UV-1601 spectrophotometer was used for UV – Vis analysis in the range of 200-800 nm.20
Results and Discussion
Flavonoids, the yellow-colored molecules are more stable than other colorants and pass over to the final stages of processing. According to Smith and Paton,1 the group of flavonoids is the most critical to sugar color being responsible for up to 30% (at pH 7) of the raw sugar color. The structural studies of plantation white sugar afford three flavonoids (1–3), they are luteolin-6-C-β-glucopyranoside (1), luteolin-7-O-ß-glucopyranoside (2), and luteolin-7-O-β-galactopyranoside (3). The Rf values, color reaction and spectral analysis (UV and NMR) of 3 analyzed flavonoids21-23 are shown in Table 1-3. These spectral results confirm that the purification method was successfully employed to isolate pure fractions of different flavonoids and were in good agreement with those previously published.21 Flavonoids 1, 2, and 3 were characterized for the first time from the sugar under investigation (Figure 1-9).
Table 1: Preliminary identification of flavonoids
|
Compound |
Rf value |
UV light |
UV/ NH3 |
|
luteolin-6-C-β-glucopyranoside |
0.43 (TBA) |
Deep purple |
Yellow-green |
|
luteolin-7-O-β-glucopyranoside |
0.16 (TBA) |
Deep purple |
Light yellow |
|
luteolin-7-O-β-galactopyranoside |
0.30 (TBA) |
Deep purple |
Yellow |
Table 2: The UV-Vis Spectral Data with Different Diagnostic Shift Reagents of Flavonoids in Plantation White Sugar
|
Compound |
Methanol |
Sodium Methoxide |
Aluminum Chloride |
Aluminum Chloride/ Hydrochloric Acid |
Sodium Acetate |
Sodium Acetate/ Boric Acid |
|
luteolin-6-C-β-glucopyranoside |
256 270sh 350 |
265 275sh 405
|
276 302sh 330 423 |
266sh 278 365 382 |
275 321 390 |
264 370 425sh |
|
luteolin-7-O-β-glucopyranoside |
254 264sh 345 |
262 298sh 392 |
273 300sh 326 430 |
275 290 355 387 |
260 265sh 403 |
260 374 |
|
luteolin-7-O-β-galactopyranoside |
254 262sh 350 |
265 300sh 392 |
272 300sh 325 |
274 290 350 385 |
260 263sh 403 |
258 372 |
Table 3: 13C NMR data of luteolin-6-C-β-glucopyranoside, luteolin-7-O-β-glucopyranoside, and luteolin-7-O-β-galactopyranoside
|
|
Compound 1 |
Compound 2 |
Compound 3 |
|
Position |
|
|
|
|
1 |
– |
– |
– |
|
2 |
164.4 |
164.5 |
166.9 |
|
3 |
102.8 |
103.1 |
104.1 |
|
4 |
182.5 |
181.9 |
184.0 |
|
5 |
160. 8 |
161.1 |
162.9 |
|
6 |
105.0 |
99.5 |
101.1 |
|
7 |
164.6 |
163.0 |
164.8 |
|
8 |
98.6 |
94.7 |
96.0 |
|
9 |
156.5 |
157.0 |
159.0 |
|
10 |
104.4 |
105.3 |
107.1 |
|
1′ |
122.4 |
121.3 |
123.4 |
|
2′ |
114.4 |
113.6 |
114.2 |
|
3′ |
146.3 |
145.8 |
147.1 |
|
4′ |
150.1 |
150.0 |
151.9 |
|
5′ |
116.1 |
116.0 |
119.2 |
|
6′ |
119.8 |
119.2 |
116.8 |
|
1g |
73.8 |
99.9 |
101.7 |
|
2g |
71.2 |
73.1 |
74.7 |
|
3g |
79.2 |
76.4 |
71.3 |
|
4g |
71.2 |
69.6 |
77.9 |
|
5g |
82.4 |
77.2 |
78.4 |
|
6g |
62.1 |
60.6 |
62.4 |
Compound 1 UV-Vis maxima 256, 270 (shoulder), 350 nm and was ascribed to luteolin-6-C-β-glucopyranoside. The 1H NMR spectra of compound 1 indicated the presence of luteolin-6-C-β-glucopyranoside, chemical shift of H-3, and H-8 at δH 6.61(1H) and 6.23 (1H, d, J=2.3). Two aromatic doublet at δH 6.82 and 7.42 (each 1H, d, J=2.3) for C-2’ and C-5’ proton and one olefinic proton at δH 7.49 for C-6’ proton and a methoxyl group at δH 3.88 that showed correlation with δ 146.33 (C-3’), 150.14 (C-4’), 160.88 (C-5), and 164.62 (C-7) bearing hydroxyl group respectively (Table 3). Spectral analysis of the known flavonoid allowed us to establish luteolin-6-C-β-glucopyranoside as the structure of compound 1.21-23
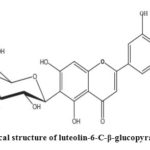 |
Scheme 1: Chemical structure of luteolin-6-C-β-glucopyranoside |
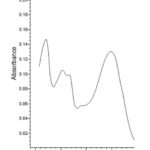 |
Figure 1: UV spectra of luteolin-6-C-β-glucopyranoside |
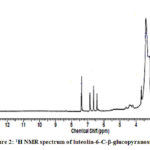 |
Figure 2: 1H NMR spectrum of luteolin-6-C-β-glucopyranoside |
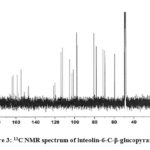 |
Figure 3: 13C NMR spectrum of luteolin-6-C-β-glucopyranoside |
Compound 2 showed UV-Vis maxima at 254, 264 (shoulder), 345 nm. This compound was tentatively assigned as luteolin-7-O-β-glucopyranoside. The results of the test for flavonoids identification of sugar are shown in Table 2. The 1H NMR spectrum of compound 2 shows an anomeric proton at δH 5.08 (1H, d, J=7.65), whereas compound 3 showed one aromatic proton at δH 5.5 (1H, d, J=7.65). The compounds 2 and 3 showed H-3 and H-6 at δH 6.61 and 6.43. 1H NMR spectrum of compounds shows doublet at 6.9 (1H, d, J=2.3Hz).
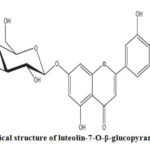 |
Scheme 2 :Chemical structure of luteolin-7-O-β-glucopyranoside |
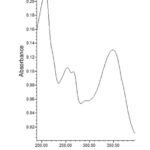 |
Figure 4: UV spectra of luteolin-7-O-β-glucopyranoside |
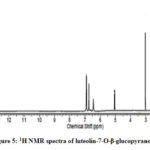 |
Figure 5: 1H NMR spectra of luteolin-7-O-β-glucopyranoside |
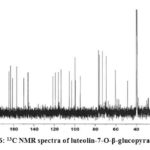 |
Figure 6: 13C NMR spectra of luteolin-7-O-β-glucopyranoside |
Compound 3 exhibited two peaks at 254 and 350 nm that were matched with the luteolin-7-O-β-galactopyranoside spectrum.21
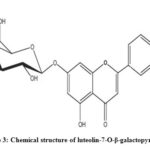 |
Scheme 3: Chemical structure of luteolin-7-O-β-galactopyranoside |
The 13C NMR signals of compound 2 and compound 3 exhibited the presence of a ketone carbonyl at δc 181.9 for luteolin-7-O-β-glucopyranoside, and 184.0 for luteolin-7-O-β-galactopyranoside. Compound 2 showed two olefinic carbons at δc 164.5 and 103.1, whereas compound 3 exhibited signals of two olefinic carbons at δc 166.9 and 104.1. The 13C NMR signals showed the presence of hydroxyl carbon position of compound 2 at 161.1 and 150.0 whereas compound 3 exhibited signals at 162.9 and 151.9 respectively.
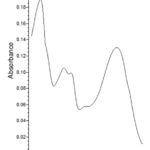 |
Figure 7: UV spectra of luteolin-7-O-β-galactopyranoside |
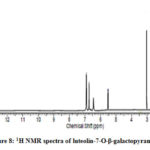 |
Figure 8: 1H NMR spectra of luteolin-7-O-β-galactopyranoside |
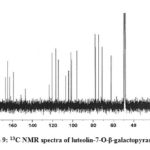 |
Figure 9: 13C NMR spectra of luteolin-7-O-β-galactopyranoside |
The data suggest that compound 2 had six sugar attachment sites at (δ 99.9, 77.2, 76.4, 73.1, 69.6, and 60.6) revealed the presence of luteolin-7-O-β-glucopyranoside. The 13C NMR chemical shifts of the sugar carbons (δ 101.7, 78.4, 77.9, 74.7, 71.3, and 62.4) showed the presence of luteolin-7-O-β-galactopyranoside (Table 3). The data allowed us to establish luteolin-7-O-β-glucopyranoside and luteolin-7-O-β-galactopyranoside as the structure of the compound by comprising of the spectral data with literature value.21-23
The presence of flavonoids in sugarcane has become a global phenomenon. Authors have reported the presence of flavones, anthocyanins, and flavonols, along with chalcones and chlorogenic acids in sugarcane and cane juice from India,20, 24-25 and abroad.26-27 Flavonoids mainly flavones enters and accumulates into the sugar crystal through the sugar processing of flavonoids containing commodities (sugarcane, cane juice, molasses, and sugar house products) and may affect sugar quality.20,28
Conclusion
The resin-based gel chromatography method developed for the identification of flavonoids was found to be simple, accurate, and sensitive. Three active flavonoids components from Indian Plantation white sugars were isolated (luteolin-6-C-β-glucopyranoside, luteolin-7-O-ß-glucopyranoside, and luteolin-7-O-β-galactopyranoside). It is concluded that sugarcane and sugar house products extract could potentially be used for food additives and the development of useful natural compounds.
Acknowledgment
The author wishes to acknowledge the support of Prof. V.K. Agrawal, Awadhesh Pratap Singh University, Rewa for this work.
Conflict Of Interest
The author declares no conflict of interest.
References
- Smith, P.; Paton, N. H. Sugarcane flavonoids. Sugar. Technol. Rev. 1985, 12,117–42.
- Yao, L. H., Jiang Y. M.; Shi J, Tomás-Barberán F. A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant. Foods. Hum. Nutr. 2004, 59(3), 113-22.
- Mc Ghie, T.K. Analysis of sugar cane flavonoids by capillary zone electrophoresis. J Chromatogr. 1993, 634, 107–12.
- Vila, F.C.; Colombo, R.; de Lira, T.O.; Yariwake, J.H. HPLC microfractionation of flavones and antioxidant (radical scavenging) activity of Saccharum officinarum L. J. Braz. Chem. Soc. 2008, 19, 903–908.
- De Armas, R.; Martinez, M.; Vincente, C.; Legaz, M.E. Free and conjugated polyamines and phenols in raw and alkaline-clarified sugarcane juice. J. Agric. Food. Chem. 1999, 47, 3086–3092.
- Colombo, R.; Yariwake, J.H.; Queroz, E.F.; Ndjoko, K.; Hostettmann, K. On-line identification of minor flavones from sugarcane juice by LC/UV/MS and post-column derivatization. J .Braz. Chem. Soc. 2009, 20,1574–1579
- Takara, K.; Ushijima, K.; Wada, K.; Iwasaki, H.; Yamashita, M. Phenolic compounds from sugarcane molasses possessing antibacterial activity against carcinogenic bacteria. J. Oleo. Sci. 2007, 56, 611–614.
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food. Chem. 2006, 99,191–203.
- Zheng, R., Su, S., Li, J., Zhao, Z., Wei, J., Fu, X., Liu, R.H. Recovery of phenolics from the ethanolic extract of sugarcane (Saccharum officinarum L.) baggase and evaluation of the antioxidant and antiproliferative activities. Ind. Crops. Prod. 2017, 107, 360–369.
- Zhao, Y., Zhu, L.C., Yu, S.J., Zhao, Z.G. HPLC-UV-ESI–MS methods for flavonoid profiling of sugarcane juice extract. Sugar. Ind. 2013, 138, 525–531.
- Farber, L.; Carpenter, F.G. Plant pigments as colorants in cane sugars’, 1972 Tech. Sess. Cane. Sugar. Refining. Res. 1975, 23-31.
- Misra, K. Mishra, C.S. Flavonoids of Saccharum officinarum flowers. Ind. J. Chem.1979, 18B, 88.
- Mishra, C.S.; Misra, K. 5-7-Dimethylapigenin 4’-0-D-glucopyranoside from Saccharum officinarum leaves. Curr. Sci. 1978, 47 (5) 152.
- Devi, K.P.; Rajavel, T.; Nabavi, S.F.; Seltzer, W.N.; Ahmadi, A. ; Mansouri, K.; Nabavi, S.M. Hesperidin: A promising anticancer agent from nature. Ind. Crops. Prod. 2015, 76, 582-589.
- Lin, Y.; Shi, R.; Wang, X.; Shen, H. Luteolin, a flavonoid with potentials for cancer prevention and therapy. Curr. Canc. Drug. Targ. 2008, 8 (7) 634-646.
- Martin, K.R. Targeting apoptosis with dietary bioactive agents. Exp. Biol. Med. 2006, 231 (2) 117-129.
- Kadam, U.S.; Ghosh, S.B.; Suprasannat, P.; Devasagayam, T.P.A.; Bapat, V.P. Antioxidant activity in sugarcane juice and its protective role against radiation-induced DNA damage. Food. Chem. 2008, 106, (3) 1154-1160.
- Zheng, R.; Su, S.; Zhou, H.; Yan, H.; Ye, J.; Zhao, Z.; You, L.; Fu, X. Antioxidant /antihyperglycemic activity of phenolics from sugarcane (Saccharum officinarum L.) bagasse and identification by UHPLC-HR-TOFMS. Ind. Crops. Prod. 2017, 101, 104-111.
- Yinrong, L.; Yeap, F. Polyphenolic constituents of blackcurrant seed residue. Food. Chem. 2003, 80, 71-76.
- Kumar, V. Isolation and identification of anticancer apigenin glycosides flavonoids from plantation white sugar. Orient. J. Chem. 2020, 36 (3) 544-549.
- Mabry, T.J.; Markham, K.R.; Thomas, M.B. The Systematic Identification of Flavonoids. (Springer-Verlag, New York 1970).
- Lu, Y.; Foo, L. Y. Polyphenolics of Salvia–a Review. Phytochem. 2002, 59 (2) 117-140.
- Kawashty, S. A.; El-Garf, I.A. The Flavonoid Chemosystematics of Egyptian Verbena Species. Biochem. Syst. Ecol. 2000, 28 (9) 919-921.
- Kumar,V.; Kumari, O.; Singh, S.; Upadhyay, R.; Tripathi, M. R. Validation of analytical method for determination of quercetin concentration in cane sugar and cane juice. Anal. Chem. An Ind. J. 2012, 11(6) 227-230.
- Kumar,V.; Kumari, O.; Singh, S.; Upadhyay, R.; Tripathi, M. R. Isolation & identification of flavonoid ‘rutin’ from Indian plantation white sugars. Int. J. Appl. Chem. 2012, 8, (1) 57-62.
- Payet, B.; Shum, A.; Smadja J. Assessment of antioxidant activity of cane brown sugars by ABTS and DPPH radical scavenging assays: Determination of their polyphenolic and volatile constituents. J. Agri. Food. Chem. 2005, 53, 10074–10079.
- Payet B, Shum Cheong Sing A, Smadja J. Comparison of the concentrations of phenolic constituents in cane sugar manufacturing products with their antioxidant activities. J. Agric. Food. Chem. 2006, 54, 7270–7276.
- Godshell, M.A.; Roberts, E.J.; New Orleans: SPRI; 1982. Phenolics in sugar products: Their role in flavour and color production. Proceedings of the 1982 Sugar Processing Research Conference. 47–72

This work is licensed under a Creative Commons Attribution 4.0 International License.









