Production and Characterization of Edible Film from Catfish (Clarias Gariepinus) Bone Gelatin Incorporated with Red Dragon Fruit (Hylocereus Polyrhizus Britton And Rose) Peel Extract
Sri Redjeki Setyawati1, Hanafi1, Nurdiani2 and Dhina Aprilia Nurani Widyahapsari1*
1Department of Quality Assurance of Food Industry of Polytechnic of AKA Bogor, Bogor 16154, Indonesia
2Department of Industrial Waste Treatment of Polytechnic of AKA Bogor, Bogor 16154, Indonesia
Corresponding Author E-mail : dhinaaprilia1488@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/360420
Article Received on : 29 June 2020
Article Accepted on : 30 Aug 2020
Article Published : 28 Aug 2020
Edible films are usually used to coat food products, so it can maintain the food quality. Gelatin is one of hydrocolloids that is used for edible film production. There are many sources of gelatin, one of them is catfish bone gelatin. In this research, catfish bones were extracted by several methods and then incorporated with red dragon fruit peel extract for edible film production. Red dragon fruit peels were extracted by maceration using distillate water. The red dragon peel extract was divided into two, one of them directly used for edible film production. The other one was evaporated until it became paste before used for edible film production. The catfish bone gelatin, red dragon fruit peel extract and edible film were analyzed for characterization. The result showed that simultaneous maceration with NaOH 0.1 N for 4 hours and 4% citric acid for 48 hours was the best method for gelatin extraction from catfish bone. Red dragon peel extract in liquid form was better than paste form when is used to modify edible film from catfish bone gelatin. The physical characteristic of edible film showed that the thickness was at 1.8 – 2.8 x 10-2 mm, the water vapor transmission rate was at 14.34-10.68 g.m-2.day-1, the solubility was at 68.7-79.8%, the elongation at break was around 8.46-12.54% and the tensile strength was at 0.1-0.58 Nm-2.
KEYWORDS:Edible Film, Catfish Bone, Gelatin, Red Dragon Fruit Peel extract
Download this article as:| Copy the following to cite this article: Setyawati S. R, Hanafi H, Nurdiani N, Widyahapsari D. A. N. Production and Characterization of Edible Film from Catfish (Clarias Gariepinus) Bone Gelatin Incorporated with Red Dragon Fruit (Hylocereus Polyrhizus Britton And Rose) Peel Extract. Orient J Chem 2020;36(4). |
| Copy the following to cite this URL: Setyawati S. R, Hanafi H, Nurdiani N, Widyahapsari D. A. N. Production and Characterization of Edible Film from Catfish (Clarias Gariepinus) Bone Gelatin Incorporated with Red Dragon Fruit (Hylocereus Polyrhizus Britton And Rose) Peel Extract. Orient J Chem 2020;36(4). Available from: https://bit.ly/3b9njS7 |
Introducton
Edible film known as thin layer film that can be eaten and usually used in food product.1 Material for edible film can be classified into 3 categories there are hydrocolloid such as protein, cellulose derivate, alginate, carrageenan, pectin, starch and other polysaccharide; lipid such as wax, acyl glycerol, fatty acid (stearate and palmitate); and combination of hydrocolloid and lipid or usually called as composite.2 One of protein source is gelatin that can be founded from skin, meat and waste of fish.3,4,5 The function of edible film can be modified by adding active substance such as antimicrobial, antioxidant, flavor enhancer, vitamin, probiotic and other substances. Dragon fruit peel has activity as antibacterial and antioxidant.6,7Red dragon fruit peel has high content of polyphenol and its antioxidant activity is higher than the red dragon fruit.8 Application of gelatin incorporated with red pitaya peel that was extracted by methanol as edible coating could extent deshelled crayfish shelf life by 2 days during refrigerated storage.9 This research wants to know the optimization process and characterization of edible film from catfish bone gelatin that was incorporated with red dragon fruit peel extract.
Materials and Methods
Materials
The materials used in this research were catfish (Clarias gariepinus ) bone from CV Raja Lele (Bogor, West Java, Indonesia), red dragon fruit peel (Hylocereus polyrhizus Britton and Rose ) from dragon fruit plant (Dramaga, Bogor, West Java, Indonesia), citric acid, hydrochloric acid, sorbitol, distillate water, sodium hydroxide, palladium cuprum.
Instruments
The instrument used in this research were Fourier-Transform Infrared (FTIR) spectrophotometer, Scanning Electron Microscopy (SEM), Magnetron Sputter Coating Instrument, mechanic tools analysis, hot plate, magnetic stirrer, spray dryer, rotary evaporator, desiccator.
Methods
Gelatin Extraction
Gelatin extraction was done by trial and error. First trial method, catfish bone was washed and then was soaked in hot water for 30 minutes to remove all of the meat. After that, catfish bone was cut into ± 2 cm and then was dried at temperature 60oC until reached the constant mass. 20 g of dried catfish bone was macerated by 100 ml of 4% hydrochloric acid solutions for 24 hours. After that, sample was washed by distillate water until pH 4. Sample was then extracted at temperature 70oC for 5 hours using hot plate. Sample was filtered and the filtrate was evaporated at 50oC for 24 hours. Second trial method was same as first method but after maceration the sample was washed by distillate water until pH 5.
Third trial method, 20 g dried cat fish bone was macerated by 4% hydrochloric acid solutions for 48 hours then was washed using distillate water until pH 6-7. Extraction process was hold at temperature 80oC for 5 hours and then the sample was filtered and the filtrate was evaporated at 80oC for 8 hours. Fourth trial method was done by same procedure as third trial method but using 4% citric acid solutions for maceration.
Fifth trial method, 20 g dried cat fish bone was soaked with 0,1 N NaOH for 4 hours to remove all of smell and fat residue then was washed by distillate water. After that, sample was macerated by 4% citric acid solutions for 48 hours then was washed by distillate water until pH 6-7. Extraction process was hold at temperature 80oC for 5 hours then was filtered and the filtrate was evaporated at 80oC for 8 hours.
The gelatin was analyzed for the percent of recovery. The % recovery was counted with equation: % Recovery =
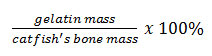
Extraction Red Dragon Fruit Peel
The red dragon fruit peel about 5 kg was washed by distillate water. Sample was cut then was dried at 50oC for 24 hours. The dried red dragon fruit peel was added by 250 ml of distillate water for maceration process along a week and every day was mixed just one time. After a week, the sample was filtered and the filtrate was divided into two batch, first batch was ready to use for the next step and the other batch was evaporated until it became paste.
Edible Film Production
About 3% (w/v) of catfish bone gelatin (gelatin from best trial method) was solved by 100 ml distillate water at temperature 60oC then was homogenized. Sorbitol amount 37.5% from gelatin powder weight was added then was homogenized for 30 minutes. The gelatin solution was added by 5% of paste or liquid red dragon fruit peel extract. Then samples were homogenized at temperature 60oC for 15 minutes. The sample was printed on a mold and dried at temperature 60oC for 8 hours.
Analysis
Edible Film Layer
Edible film layer was analyzed using method by Aleman10, and was measured using micrometer at 6 different part of edible film then the average was counted.
Water Vapor Transmission Rate
Water vapor transmission rate was analyzed using method by Gontard11.
Percent Solubility
Analysis for percent solubility was analyzed using method by Gontard11 that was modified. Edible film was cut into 2 x 2 cm sized and then together with filter paper was dried at temperature 105oC for 24 hours. Edible film and filter paper were weight and noted as initial weight. Edible film was soaked and mixed into 50 ml distillate water for 6 hours. After that, it was filtered using filter paper, the pellet and filter paper were dried at temperature 105oC for 24 hour and then was weight as final weight. The percent solubility was count using equation: Percent Solubility =
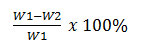
W1 : initial weight
W2 : final weight
Morphology
Morphology of edible film was analyzed using Scanning Electron Microscope (SEM). Edible film was coated with palladium cuprum using Magnetron Sputter Coating Instrument. Beside morphology, the thin layer, porosity and pore size were also analyzed.
Tensile Strength and Elongation
The tensile strength was analyzed using texture analyzed and ASTM D882-09 (2009) as the guideline.
Fourier-Transform Infrared (FTIR) Spectroscopy
The commercial gelatin, catfish bone gelatin, red dragon fruit peel extract and edible film was analyzed by Fourier-Transform Infrared (FTIR) Spectroscopy.
Result and Discussion
Gelatin Extraction
As shown in table 1, % recovery of gelatin that was extracted by fifth trial method or simultaneous macerated with NaOH 0.1 N for 4 hours and 4% citric acid solutions for 48 hours was higher than other method. Beside that gelatin that was macerated by citric acid has better appearance than gelatin that was macerated by hydrochloric acid (See Fig 1). The gelatin extraction yields were strongly related to the stability of collagen structure. In general, the acid pretreatment destabilizes the cross-links, leading to the loss of ordered structure of natural collagen and facilitates the extraction of gelatin12. Compared to hydrochloric acid, citric acid was more efficient for extracting gelatin. According to Niu,13 citric acid can disrupt more cross-links of collagen molecules than hydrochloric acid. The multiple ionizable groups in citric acid have the ability to ionize a large amount of reactive hydrogen ions at low concentration to disrupt the cross-links of collagen molecules and subsequently facilitate the extraction rate of gelatin.
Table 1: Percent Recovery of Gelatin Extraction from Catfish Bone
|
Gelatin Extraction Method |
% Recovery |
|
First trial method Second trial method Third trial method Fourth trial method Fifth trial method |
0,5 % 0,9 % 1,5 % 2,5 % 5.06 % |
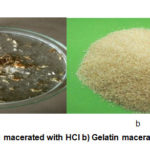 |
Figure 1: a) Gelatin macerated with HCl b) Gelatin macerated with citric acid |
Edible Film Production
Edible film that was made from catfish bone gelatin incorporated with red dragon peel extract in paste form has sticky texture and hard to lift from the mold (see Fig 2). Meanwhile, the edible film from catfish bone gelatin mixed with red dragon fruit extract in liquid form has good appearance (see Fig 3). The sticky texture of edible film was caused by the pectin content of red dragon peel extract. Concentration of pectin increased after the red dragon peel extract was evaporated. Pectin was an anionic polysaccharide containing active carboxyl groups that can undergo ionic interaction with the negatively charged gelatin chains. This ionic interaction produced reversible physical hydrogel with homogenous molecular arrangement.14
|
|
Figure 2: Edible Film from Catfish Bone Gelatin Incorporated with Red Dragon Fruit Peel Extract in Paste Form |
|
|
Figure 3: Edible Film from Catfish Bone Gelatin Incorporated with Red Dragon Fruit Peel Extract in Liquid Form |
Edible film from best method production was then characterized. According to table 2, the physical measurement of edible film showed that the thickness was at 1.8 – 2.8 x 10-2 mm, the water vapor transmission rate was at 14.34-10.68 g.m-2.day-1, the solubility was at 68.7-79.8%, the elongation at break was around 8.46-12.54% and the tensile strength was at 0.1-0.58 Nm-2.
Table 2: Water Vapor Transmission Rate, Solubility, Elongation and Tensile Strength of Edible Film from Catfish Bone Gelatin Incorporated with Red Dragon Fruit Peel Extract in Liquid Form
|
Thickness (mm) |
WVTR (g.m-2.day-1) |
Solubility (%) |
Elongation (%) |
Tensile Strength (Nm-2) |
|
1.80 x 10-2 |
14.34 |
68.70 |
8.46 |
0.10 |
|
2.20 x 10-2 |
13.29 |
75.10 |
10.88 |
0.34 |
|
2.80 x 10-2 |
10.68 |
79.80 |
12.54 |
0.58 |
SEM of Edible Film from Catfish Bone Gelatin Incorporated with Red Dragon Fruit Peel Extract in Liquid Form
Result of Scanning Electron Microscopy (SEM) of edible film was shown at Fig 4, The SEM result showed that edible films have straight structure without pore, so water vapor cannot penetrate into the edible film. The thickness has no impact on the SEM result of edible film.
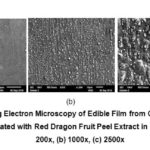 |
Figure 4: Scanning Electron Microscopy of Edible Film from Catfish Bone Gelatin that Incorporated with Red Dragon Fruit Peel Extract in Liquid Form. |
FTIR of Commercial Gelatin, Catfish Bone Gelatin, Red Dragon Fruit Peel Extract and Edible Film
As shown at Fig.5, commercial gelatin has two sharp and clear bend. First at 3272 cm-1 as functional group of O-H and at 1636 cm-1 as functional group of C-H. Besides that, there was stretching bend at 1095 cm-1 as functional group of C-O.
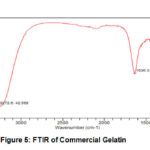 |
Figure 5: FTIR of Commercial Gelatin |
FTIR of catfish bone gelatin was shown at Fig 6, Catfish bone gelatin has some high intensity and sharp bend. There were functional group of O-H at 3313 cm-1; CH at 2922 cm-1, 1461 cm-1 and 909 cm-1; C=C at 1640 cm-1; and C-O at 1084 cm-1. This result indicated that catfish bone gelatin has several functional groups similar to commercial gelatin. This result was different from another research about gelatin catfish bone by Nuryanto15, that claimed catfish bone gelatin has functional group of C=O, C-N, =C-N, NH and CH2.
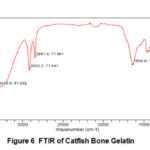 |
Figure 6: FTIR of Catfish Bone Gelatin |
FTIR of red dragon fruit peel extract was shown at Fig 7. There were only two bends with high and clear intensity, first at 3265 cm-1 as functional group of O-H and at 1628 cm-1 as functional group of C=C. Vijayakumar16 said that red dragon fruit extract has kind of chemistry bond such as O-H, C-H, C=O, CH3, C-O-C.
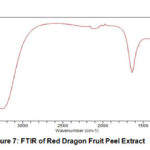 |
Figure 7: FTIR of Red Dragon Fruit Peel Extract |
FTIR of edible film from catfish bone gelatin that was incorporated with red dragon fruit peel extract showed that at 3261 cm-1 had an O-H functional group, 1636 cm-1 as C=C functional group, 1416 cm-1 as CH functional group. The last three peak at 1151 cm-1, 1077 cm-1, 1017 cm-1 were C-O functional group (see Fig 8). Some of these functional groups were identical to those of the red dragon fruit peel extract. That means, characteristics of red dragon fruit peel extract were still in edible film.
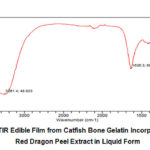 |
Figure 8: FTIR Edible Film from Catfish Bone Gelatin Incorporated with Red Dragon Peel Extract in Liquid Form |
Conclusion
Catfish bone gelatin incorporated with red dragon peel extract in liquid form has the potential for edible film. This edible film had a straight structure without pores and the characteristics of red dragon fruit peel extract were still in edible film. The physical properties of this edible film showed that a thickness of 1.8 – 2.8 x 10-2 mm, Water Vapor Transition Rate (WVTR) 14.34-10.68 g.m-2.day-1, solubility 68.7-79.8%, elongation at about 8.46-12.54% and tensile strength 0.1-0.58 Nm-2.
Acknowledgement
This research was funded by Industrial Human Resource Development Agency, Ministry of Industry of Indonesia.
Conflict of Interest
The authors declare no conflicts of interest.
Funding Source
This research was funded by Industrial Human Resource Development Agency, Ministry of Industry of Indonesia.
References
- Krochta, J.M.; Baldwin, E.A.; and Nisperos-Carriedo, M.O (Eds). Technomis Publishing Co. Inc, Pennsylvania. 1994.
- Shit, S.Cand Shah, P.M. J Polym. 2014.
CrossRef - Yang, H.;Wang, Y.; Jiang, M.; Oh, J.; Herring, J.; Zhou, P. J Food Sc 2007, 72(4), 188-195.
CrossRef - Gómez-Guillén, M.C.; Pérez-Mateos, M.; Gómez-Estaca, J.; LópezCaballero, E.; Giménez, B.; Montero, P. Trends Food Sci Technol, 2009, 20, 3-16.
CrossRef - Feng, X.; Ng, V.K.; Mikš-Krajnik, M.; Food Bioproc Tech. 2017,1(1), 89-102.
CrossRef - Nurmahani, M.M.; Osman, A.; Abdul Hamid, A.; Mohamad Ghazali, F.; Pak D.S. Int Food Res J. 2012, 19(1), 77-84.
- Nurliyana, R.; Syed Zahir, I.; Mustapha S, K.; Aisyah, M.R.; Kamarul, R. Int Food Res J. 2010, 17, 367-375.
- Wu,C.; Yun, C.; Hsu, H.W.; Chiu, C.C. J Food Chem. 2006, 95, 319–327.
CrossRef - Liu, W.; Shen, Y.; Li, N.; Mei, J.; Xie, J. J Food Qual. 2019, 1-8
CrossRef - Alemán,A.; Blanco-Pascual, N.; Montero,M.P.; Gόmez-Guillén, M. J Food Hydrocoll. 2016, 56, 277-284.
CrossRef - Gontard, N.; Guilbert, S.; Cuq, J. J Food Sci. 1992, 57(1), 190-195.
CrossRef - Benjakul, S.; Thiansilakul, Y.; Visessanguan, W.; Roytrakul, S.; Kishimura, H.; Prodpran, T.; Meesane, J. Sci. Food Agric. 2010, 90, 132–138.
CrossRef - Niu, L.; Zhou, X.; Yuan, C.; Bai, Y.; Lai, K.; Yang, F.; Huang, Y. Food Hydrocoll. 2013, 33, 336–341.
CrossRef - Farris, S.; Schaich, K.M.; Liu, L.S.; Cooke, P.H.; Piergiovanni, L.; Yam, K.L. Food Hydrocoll. 2011, 25, 61-70.
CrossRef - Nuryanto, R.; Trisunaryanti, W.; Falah, I.I.; Triyono. IOP Conf. Series: Mater Sci Eng. 2018, 349, 012051.
CrossRef - Vijayakumar, R.; Gani, S.S.A.; Zaidan, U.H.; Halmi, M.I.E. App Sci. 2018, 8, 1516-1536.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.











