Biofabrication of Zinc Oxide Nanoparticles using the Isolated Flavonoid from Combretum ovalifolium and its Anti-oxidative Ability and Catalytic Degradation of Methylene Blue Dye
G. Dayana Jeyaleela* , J. Rosaline Vimala
, J. Rosaline Vimala , S. Margrat Sheela
, S. Margrat Sheela , A. Agila
, A. Agila , M. Stella Bharathy
, M. Stella Bharathy and M. Divya
and M. Divya
Department of Chemistry, Holy Cross College (Autonomous), Affiliated by Bharathidasan University, Tiruchirappalli-620 002, Tamilnadu, India.
Corresponding Author E-mail : princedayana1221@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/360409
Article Received on : 07 July 2020
Article Accepted on : 08 Aug 2020
Article Published : 25 Jul 2020
Medicinal plants engrossed great attention for the synthesis of new chemical entities and its biomolecules has fabulous application in many fields such as corrosion, nanotechnology, pharmaceutical companies, and cosmetic industries. The main purpose of the present work is biosynthesis of zinc oxide nanoparticles via simple homogeneous precipitation method using the isolated flavonoid quercetin, which was derived from the medicinal plant Combretum ovalifolium. Antioxidant activity and photocatalytic degradation of methylene blue dye were studied on the synthesized ZnONPs. Zinc acetate dihydrate (Zn (CH3COO)2.2H2O) was utilized as a precursor and quercetin as a reducing agent. Formed Quercetin mediated zinc oxide nanoparticles (Q-ZnONPs) were characterized by UV-Visible, FT-IR, XRD, EDX, and SEM. Fiber shaped Q-ZnONPs were obtained with single crystalline nature, its average diameter was found to be 31.24nm. It is reported that, 168.045μg/ml of Q-ZnONPs scavenged 50% of DPPH on antioxidant activity. The degradation behaviour of the nanomaterial was analysed. The results showed that on the addition of biosynthesized Q-ZnONPs, the degradation of methylene blue increased up to 76.01% at 240 minutes.
KEYWORDS:Antioxidant activity; Biosynthesis of ZnONPsI; Characterization; Degradation of Methylene Blue; Synthesis Mechanism; Quercetin; Zinc acetate dihydrate
Download this article as:| Copy the following to cite this article: Jeyaleela G. D, Vimala J. R, Sheela S. M, Agila A, Bharathy M. S, Divya M. Biofabrication of Zinc Oxide Nanoparticles using the Isolated Flavonoid from Combretum ovalifolium and its Anti-oxidative Ability and Catalytic Degradation of Methylene Blue Dye . Orient J Chem 2020;36(4). |
| Copy the following to cite this URL: Jeyaleela G. D, Vimala J. R, Sheela S. M, Agila A, Bharathy M. S, Divya M. Biofabrication of Zinc Oxide Nanoparticles using the Isolated Flavonoid from Combretum ovalifolium and its Anti-oxidative Ability and Catalytic Degradation of Methylene Blue Dye . Orient J Chem 2020;36(4). Available from: https://bit.ly/3g5SfEt |
Introduction
Quercetin is a flavonoid compound (polyphenol) which can be easily isolated from plant sources. Quercetin being accepted in the phase I clinical trial against the cancer studies, has shown to be effective towards oxidative injury and cytotoxicity. However, due to its poor water solubility, instability and permeability it has limited applications in pharmaceutical field. Quercetin has limited applications in Alzheimer’s diseases owing to its difficulty in crossing BBB adsorption (blood–brain-barrier) and poor oral bioavailability.1,2 To enhance its solubility and exert its bioactivity, novel preparations (encapsulation) such as liposomes, polymers, micelles and nanoparticles derived quercetin were developed. Since polymer materials (encapsulation) are not stable in long term storage, nanotechnology methods (complexation with metals) are suggested for preparation of quercetin based drugs especially in the cancer therapy treatments.3-6
Nanotechnology is blaze technology. It involves the synthesis and utilization of materials in nanoscale dimension (nanoparticles). It is a bridge between bulk and molecular or atomic structures; with size dependent property being observed. Due to nanomaterials specific optical property, unusual mechanical property, electronic property, high catalytic activity, and low melting point, synthesis of nanomaterials has got the scope in the modern science.7,8 Different physical and chemical methods exists for the synthesis of a large number of nanomaterials and among all, the most widely used methods are chemical precipitation method, simple solution-based method, sol-gel method, hydrothermal/solvothermal, photochemical and electrochemical reduction.9 Physical methods possess certain drawbacks such as use of costly equipment, large space for setting up machines, high pressure and temperature. Correspondingly in chemical methods, toxic chemicals are used in the synthesis which exhibits toxic effects on its applications, especially in medicinal fields. In both physical and chemical methods addition of stabilizing agents are needed for the synthesis.10-12 Alternatively, a green approach is established in a recent decade which has more attention for the synthesis of nanoparticles in an eco-friendly and inexpensive way.
Biosynthesis of nanoparticles is an alternative approach to the synthesis of nanoparticles using plant extracts, fungi, bacteria, yeast, algae, etc.13,14 Plant extracts mediated nanoparticles approach is most biocompatible, effective, safe and low-cost process than other methods. Furthermore, plant extract also acts as stabilizing and reducing agents in nanomaterials synthesis.15 Literature states that plant extract are successfully used to synthesize nanoparticles such as copper, silver, gold, cobalt, platinum, palladium, magnetite and zinc.16 Plant extracts mediated nanomaterials have an effective remedy for cancer, hepatitis, malaria, and other acute diseases, even though, plant extract mediated nanomaterials has excess of additional impurities which are due to numerous phytocompounds present in the plant extracts which can affect the shape, size, and property of the resulted nanomaterials.17,18
Zinc oxide nanoparticles have the potential to reduce (or) eliminate the toxic chemicals such as sulfur, arsenic from water as well as it degrades the organic dyes in the polluted wastewater.19 Although ZnO employed for drug delivery, it still has some limitation in the medicinal field due to its cytotoxicity effect which is not yet to be resolved.20
To overcome the above drawback of the existing chemical, physical and green methods, present work focused on the synthesis of zinc oxide nanomaterials using specific bioactive flavonoid which is isolated from medicinal plant. Since bioactive flavonoid is used, it may reduce the toxicity of the resulted zinc oxide nanomaterial. Also it can control the reaction (size & shape) and minimize the impurity of nanomaterials. Using the biosynthesized zinc oxide nanoparticles, antioxidant activity and photocatalytic degradation of methylene blue dye were studied.
Materials and Methods
Materials
Precursor zinc acetate dihydrate, DPPH, methylene blue, double distilled water and methanol(Merck product)were purchased from Ponmani & Co biochemicals suppliers, Trichy, India. Flavonoid quercetin was previously isolated and characterized from the medicinal plant Combretum ovalifolium.21
Biofabrication of Quercetin Mediated Zinc Oxide Nanoparticles (Q-ZnONPs)
0.01M concentration of quercetin was prepared by dissolving 0.302236g of quercetin using 100ml methanol in separate standard flask. Initially, 0.18348g of Zinc acetate dihydrate was taken in a conical flask followed by the addition of 100ml of double-distilled water and then placed on a magnetic stirrer at 600C. After the dissolution of zinc acetate, 10ml of quercetin was added drop wise then allowed to react. After that, every 30 minutes interval 10ml of quercetin was added drop wise. After 7 hrs, the whole solution was taken from the magnetic stirrer and allowed to settle overnight. Formed zinc oxide nanoparticles were collected by centrifugation at 6000rpm and final residue were washed with double distilled water followed by methanol. Collected Zinc oxide nano particles were dried in a hot air oven for the complete conversion of Zn(OH)2 into quercitin mediated zinc oxide nanoparticles (Q-ZnONPs). 22-24
Characterization of Q-ZnONPs
Formed zinc oxide nanoparticles were initially confirmed by UV-Visible spectroscopy and a small portion of ZnONPs were taken for the FT-IR analysis in the range of 500 cm-1to 4000cm-1 to identify the functional groups involved in its synthesis by comparing it with the spectra of quercetin. ZnONPs were mounted on aluminium stubs using carbon tape further sputtered with gold to study the surface analysis at low and high magnifications with the help of SEM. Obtained ZnONPs were crushed and made it into powder to examine the crystalline phase of the crystals by XRD analysis under Cu-Kα with λ -1.54056, Ni filter of Bragg’s angles 10o<2θ<80o at room temperature. Elemental composition in the ZnONPs was analyzed with the help of EDX.25-27
Antioxidant Activity of Q-ZnONPs
0.3 mM concentration of DPPH reagent was prepared by dissolving 11.82 gms of DPPH in 100 ml of methanol. Obtained Q-ZnONPs were mixed with 1ml of methanol. From that, 50µg/ml, 100µg/ml, 150µg/ml and 200µg/ml concentrations were prepared. To the 1ml of each different concentration 5ml of DPPH reagent was added separately and kept in a dark room for thirty minutes. Then, the absorbances of the samples were recorded at 517nm and the percentage of radical scavenging activity i.e anti-oxidant activity was calculated by following standard formulae-1. Control reading was recorded by taking 5 ml of DPPH reagent with 1ml of methanol.28-30
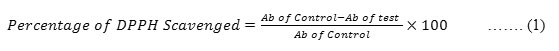
Ab of control – Control’s absorbance, Ab of test- Test solution’s absorbance
The IC50 values were calculated by linear regression of plots, where the abscissa represents the concentration of the tested sample and the ordinate, the average percent of radical scavenging activity.
Evaluation of the Degradation Property of Biosynthesized Q-ZnONPs on Methylene Blue Dye
The catalytic behaviour of the nanomaterial in the reduction of methylene blue dye was evaluated after every 30 minutes interval by taking 3ml of test sample (methylene blue of 0.1M) treated with 500µg/ml of Q-ZnONP placed on a magnetic stirrer. The reaction progress was noted by recording its absorbance using UV-Visible spectrophotometer. The percentage removal was calculated with the help of the standard calibration curve obtained by measuring the absorbance of untreated methylene blue of varied concentrations(0.1M to 1M) using the formula-2. 31,32
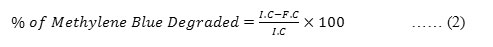
Where,
I.C– Initial Concentration of Methylene blue
F.C– Final (Sample) Concentration of Methylene blue
Results and Discussion
Characterization of Isolated Flavonoid
Flavonoid quercetin was previously isolated from the leaf and stem parts of Combretum ovalifolium. Then its structure was characterized with the help of spectroscopic methods.33
Mass Spectrum of Quercetin
Mass spectrum of quercetin is shown in fig -1 and it clearly explains that the major parent molecular ion (M+1) peak appeared at m/z=302 and it is further fragmented at m/z=285, 257,229, 201, 165, 153, 137 and 30.
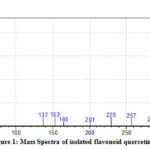 |
Figure 1: Mass Spectra of isolated flavonoid quercetin |
NMR Spectra of Isolated Quercetin
1H & 13C-NMR spectra of isolated flavonoid quercetin are shown in fig-2 and 3.The following data confirms its structure.
1H NMR: δ 9.335 (OH in C3,s), 12.512 (OH in C5,s), 6.203-6.198 (1H in C6,dd), 10.810 (OH in C7,s), 6.424-6.419 (1H in C8,d), 7.695-7.690 (1H in C2’, dd), 6.909-6.888 (1H in C3’,dd), 9.623 (OH in C4’,s), 9.393 (OH in C5’,s), 7.568-7.542 (1H in C6’.dddd).
13C NMR: δ 145.402 (C2), 136.172 (C3), 176.263 (C4), 161.144 (C5), 98.607 (C6), 164.304 (C7), 93.781 (C8), 103.440 (C9), 156.559 (C10), 122.385 (C1’), 120.410 (C2’), 116.034 (C3’), 148.124 (C4’), 147.210 (C5’), 115.476 (C6’).
 |
Figure 2: 1H-NMR of spectra of isolated flavonoid quercetin |
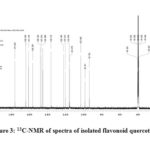 |
Figure 3: 13C-NMR of spectra of isolated flavonoid quercetin |
The molecular formula of the isolated compound is C15 H10 O7, accurate molecular weight is found to be 302.236 and name of the isolated flavonoid is flavone-3-rutinoside (quercetin). Numbering and structure of the isolated flavonoid quercetin is shown in the fig-4.
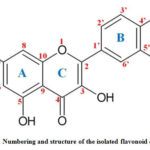 |
Figure 4: Numbering and structure of the isolated flavonoid quercetin |
Characterization of ZnONPs
Formation of zinc oxide nanoparticles was preliminarily confirmed by colour changes before and after the addition of reducing agent (quercetin). 34 Initially (in fig-5 before addition), the colour of the isolated quercetin is pale yellow, on adding the zinc acetate solution the colour changes from pale yellow to yellow (stage-1). When time extends the colour of the solution mixer gets darkened and became colloidal which is shown from stage-2 to stage-4. This colour transformation of quercetin with zinc acetate solution is due to the vibration of surface plasmon resonance of zinc.
 |
Figure 5: Various stages of formation of Q-ZnONPs |
UV-Visible of Biosynthesized Q-ZnONPs
Generally, flavonoids show two absorption maxima in UV-Visible spectra, one of which occurs in the range 240-285nm (band-II for ring B cinnamoyl system) and the other in the range 300-400nm (band-I for ring A benzoyl system). Fig-6 shows the UV-Visible spectra of quercetin before and after synthesis of Q-ZnONPs. Spectral results shows that, before reaction quercetin exhibits λmax at 298nm, and 354nm which are then shifted to 374nm and 457nm after formation of ZnONPs. It is therefore evidenced that rings A and B of quercetin are involved in the reduction of Zn2+ to Zn0. 35,36
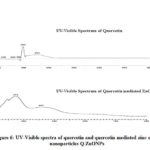 |
Figure 6: UV-Visible spectra of quercetin and quercetin mediated zinc oxide nanoparticles Q-ZnONPs |
FT-IR Spectra of Biosynthesized Q-ZnONPs
FT-IR spectra of quercetin and quercetin mediated ZnONPs (Q-ZnONPs) is ascertained from the fig-7 and the resulted spectral data reveals that quercetin has the functionalities of OH (3409cm-1‑), C-H (2794- 2712 cm-1), C=O (1806 cm-1), C=C (1611-1522 cm-1) and C-H (1014 cm-1) bending vibrations. In the case of quercetin mediated ZnONPs, OH frequency occurred at 3427cm-1, C-H, C=O, C=C, and C-H bending vibrations at 2987-2910 cm-1, 1600-1203 cm-1, and 1064 cm-1 correspondingly. Multiple peaks appeared at 600 – 400 cm-1 which are responsible for metal-oxygen bonding in quercetin mediated ZnONPs and shift in C=O and suppression of OH frequencies confirm the formation of bonding between zinc and quercetin.37
 |
Figure 7: FT-IR spectra of Quercetin and Quercetin mediated Zinc oxide nanoparticles |
XRD of Results of Biosynthesized Q-ZnONPs
Fig-8 shows the XRD results of quercetin mediated ZnONPs which gives several Bragg reflections. These are indexed based on the wurtzite structure of zinc oxide. Diffraction peaks of quercetin mediated zinc oxide nanoparticles found at (100), (002) and (101) plane are closer to the reported values of JCPDS standard card no: 36-1451. The average size of the particles was calculated by Debye-Scherrer’s equation; the average size of the quercetin mediated ZnONPs is 31.24nm. 38,39
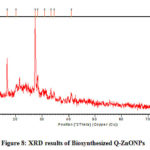 |
Figure 8: XRD results of Biosynthesized Q-ZnONPs |
EDX Analysis of Biosynthesized Q-ZnONPs
The EDX of biosynthesized quercetin mediated ZnONPs was done to identify the elemental composition present in it. Fig-9 reveals zinc and oxygen elements are mostly present in the quercetin mediated zinc oxide nanoparticles along with the presence of little impurities which can be seen.
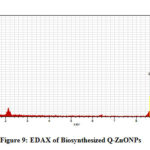 |
Figure 9: EDAX of Biosynthesized Q-ZnONPs |
SEM Images of Biosynthesized Q-ZnONPs
Morphology of the synthesized quercetin mediated ZnONPs were analyzed with the help of SEM imaging. Quercetin mediated ZnONPs are single crystalline in nature. This is because even, quercetin act as reducing and stabilization agent along with that they act as a capping reagent so that it controls the shape and size of the zinc nanomaterials (uniformly). Quercetin mediated zinc oxide nanomaterials shows zinc nano fiber type morphology which is shown in fig-10. 40,41
 |
Figure 10: SEM magnification Image of Biosynthesized Q-ZnONPs |
Possible Complexation and Mechanism of Q-ZnONPs with Spectral Evidences
Schematic diagram figure-11 explains the common possible intra molecular mechanism of nanoparticles formation. Zinc oxide nuclei initially formed (nucleation formation) subsequently either oriented aggregation or random aggregation developed. After forming aggregation the formed nanoparticles can assemble themselves (oriented aggregation) and forms uniform structure (or) diverse morphology/shapes (random aggregation).
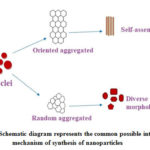 |
Figure 11: Schematic diagram represents the common possible intra molecular mechanism of synthesis of nanoparticles |
Similarly obtained Q-ZnONPs followed the oriented aggregation mechanism which is given in figure-12. In the first step in order to activate the zinc acetate-quercetin reaction mixture was heated at 60oC temperature with constant stirring. Second step zinc element forms complex with quercetin C=O of C-4 carbon and OH of C-5, C-3, C-4’ and C-5’ (in fig-4). From the UV-Visible spectral report ring A and B involves in the bonding which has hydroxyl groups and it can easily form M-O bond with zinc element. In the same way FT-IR spectral results also confirms the functionalities of C=O and –OH are involved in the synthesis which is present in A, B and C rings of quercetin.
Growth aggregation i.e oriented aggregation has occurred in the third step, then Q-ZnONPs assemble itself and form uniform fiber shaped nanomaterials which are formed in the slow process. From the SEM image figure-13 the individual fiber in the beam is seen. At fourth step an individual fibers are aggregated and forms the beam like shape which is shown in figure-14. Final step (V) is fast step in that all the nanoparticles are accumulated and forms large size particles (in figure-15). Accumulated particles can be separated individual fiber by crushing it.
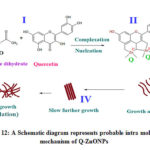 |
Figure 12: A Schematic diagram represents probable intra molecular mechanism of Q-ZnONPs |
 |
Figure 13: Step-III image (growth aggregation image) of biosynthesized Q-ZnONPS |
 |
Figure 14: Step-IV image (slow further growth) of biosynthesized Q-ZnONPS |
 |
Figure 15: Step-V image (Fast final growth) of biosynthesized Q-ZnONPS |
Antioxidant Activity of Biosynthesized Q-ZnONPs
Table-1 shows the antioxidant activity of biosynthesized quercetin mediated ZnONPs. Inhibition percentage of DPPH (antioxidant activity) on quercetin mediated ZnONPs increased with increasing concentration of synthesized ZnONPs which is shown in fig-16. Moreover, the absorbance values of synthesized ZnONPs get decreased with increasing its concentration. IC50 value of quercetin mediated ZnONPs is 168.045µg/ml.
Table 1: Antioxidant activity of Biosynthesized Q-ZnONPs
|
S.No. |
Concentration of Q-ZnONPs (µg/ml) |
Control OD |
Sample OD (Q-ZnONPs) |
Inhibition % of Q-ZnONPs |
|
1. |
50 |
1.73 |
1.444 |
16.53 |
|
2. |
100 |
1.73 |
1.193 |
31.04 |
|
3. |
150 |
1.73 |
0.969 |
43.99 |
|
4. |
200 |
1.73 |
0.700 |
59.55 |
|
IC50 Values |
Qu- ZnONPs is = 168.045µg/ml |
|||
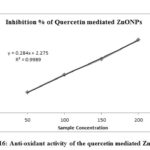 |
Figure 16: Anti-oxidant activity of the quercetin mediated ZnONPs |
Photocatalytic Degradation of Methylene Blue Dye by using Biosynthesized Q-ZnONPs
Zinc oxide nanomaterials have great application in the reduction of organic dyes as well as degradation of toxic metals and chemicals. Standard graph of methylene blue is shown in fig-17. Untreated and Q-ZnONPs treated methylene blue dye UV-Visible spectral results is shown in fig-18. Spectral results clearly explain that untreated methylene blue shows maximum absorbance, while Q-ZnONPs treating methylene blue exhibits lower absorbances which is shown in fig-15. Quercetin mediated zinc oxide nanoparticles (Q-ZnONPs) suppressed or degraded 76.01% of methylene blue at 240mins (Table-2).
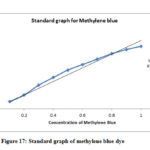 |
Figure 17: Standard graph of methylene blue dye |
Table 2: Results of percentage of degradation of methylene blue dye by Q-ZnONPs
|
S.No |
Time in Minutes |
Percentage of Degradation of Methylene blue by Q-ZnONPs |
|
1 |
0 |
0 |
|
2 |
30 |
11.37 |
|
3 |
60 |
22.10 |
|
4 |
90 |
35.53 |
|
5 |
120 |
47.16 |
|
6 |
150 |
58.62 |
|
7 |
180 |
68.13 |
|
8 |
210 |
70.74 |
|
9 |
240 |
76.01 |
 |
Figure 18: UV-Spectrum of untreated and Q-ZnONPs treated methylene blue |
Conclusion
Above study explore that biomolecule especially flavonoids from plant source can be used in the synthesis of nanomaterials. This bioreductive approach is simple, effective and of ecofriendly nature. Usage of biomolecules in ZnONPs synthesis can be an alternative process for reducing the toxicity of zinc in medicinal applications. Addition of stabilizing and capping reagents might not be needed while using natural flavonoid. Quercetin plays the role of reducing, stabilizing and capping reagents. The results summarized that quercetin mediated ZnONPs were successfully synthesized by simple homogeneous precipitation method and characterized by UV-Visible spectroscopy, FT-IR, XRD, EDX, and SEM analysis. Biosynthesized zinc oxide nanoparticles are single crystalline nature as well as zinc fiber type zinc oxide seeds and its probable mechanism are first time reported in bioreduction method. Because of the free radical scavenging property of Q-ZnONPs, it can be used in free radical diseases like cancer, asthma, diabetes and inflammatory joint diseases. Owing to its catalytic activity of dye degradation, Q-ZnONPs may be applicable in dye industries for wastewater treatment.
Acknowledgment
We thank our professor Dr. T. Pazhanivel, Assistant Professor, Smart materials Interface Laboratory, Department of Physics, Periyar University, Salem, Tamilnadu, India, and his research scholar Ms. K. Bhuvaneswari, who constantly provide facility to carry out the photocatalytic activity of my nanoparticles in his institute. We thank our institute and Principal Dr. Sr. Christina Bridget, Holy Cross College (Autonomous), Tiruchirappalli, India, for providing laboratory facility to perform our research work.
Conflicts of Interest
The authors have declared no conflicts of interest.
References
- Fahimeh Aghapour.; Ali Akbar Moghadamnia.; Andrea Nicolini.; Seydeh Narges Mousavi Kani.; Ladan Barari.; Payam Morakabati.; Leyla Rezazadeh.; Sohrab Kazemi. Quercetin conjugated with silica nanoparticles inhibits tumor growth in MCF-7 breast cancer cell lines. Biochem. Bioph. Res. Co. 2018, 1-6.
- Ansari, M.A.; Abdul, H.M.; Joshi, G.; Opii, W. O.; Butterfield, D. A. Protective Effect of Quercetin in Primary Neurons Against Abeta (1-42): Relevance to Alzheimer’s Disease. J. Nutr. Biochem. 2009, 20, 269–275.
- Anandam, S.; Selvamuthukumar, S. Fabrication of cyclodextrin nanosponges for quercetin delivery: physicochemical characterization, photostability, and antioxidant effects. J. Mater. Sci. 2014, 49, 8140–8153.
- Kakran, M.; Sahoo, N. G.; Li, L. Dissolution enhancement of quercetin through nanofabrication, complexation, and solid dispersion. Colloids. Surf. B. 2011, 88,121–130.
- Mukhopadhyay, P.; Prajapati, A. K. Quercetin in anti-diabetic research and strategies for improved quercetin bioavailability using polymer-based carriers – a review. RSC Adv. 2015, 5, 97547–97562.
- Wu, H.; Yang, R.; Song, B.; Han, Q.; Li, J.; Zhang, Y.; Fang, Y.; Tenne, R.; Wang, C.; Biocompatible Inorganic Fullerene-Like Molybdenum Disulfide Nanoparticles Produced by Pulsed Laser Ablation in Water. ACS Nano. 2011, 5, 1276–1281.
- Barve, A.K.; Gadegone, S. M.; Lanjewar, M. R.; Lanjewar, R. B. Synthesis of ZnO Nanomaterial by Precipitation Method and its Characterization. Int. J. Chem. Phys. Sci. 2015, 4, 432-439.
- Hasnidawani, J.N.; Azlina, H.N.; Norita, H.; Bonnia, N.N.; Ratim, S,; Ali, E.S. Synthesis of ZnO Nanostructures Using Sol-Gel Method. Procedia. Chem. 2016, 19, 211 – 216.
- Surabhi Siva Kumar.; Putcha Venkateswarlu.; Vanka Ranga Rao.; Gollapalli Nageswara Rao. Synthesis, characterization and optical properties of zinc oxide nanoparticles. Int. Nano. Lett. 2013, 3:30.
- Gnanasangeetha, D.; SaralaThambavani, D. One Pot Synthesis of Zinc Oxide Nanoparticles via Chemical and Green Method, Res. J. Mater. Sci. 2013, 1, 1-8.
- Jafar Ahamed, A.; Vijaya Kumar, P. Synthesis and characterization of ZnO nanoparticles by co-precipitation method at room temperature. J. Chem. Pharm. Res. 2016, 8, 624-628.
- Lijuana, An.; Wang Junb.; Zhang Tiefengc.; Yang Hanlin.; Sun Zhihui. Synthesis of ZnO Nanoparticles by Direct Precipitation Method. Adv. Mater. Res. 2012, 380, 335-338.
- Shabnam Fakhari.; Mina Jamzad.; Hassan Kabiri Fard. Green synthesis of zinc oxide nanoparticles: a Comparison. Green. Chem. Lett. Rev. 2019, 12:1, 19-24.
- Vidya, C.; Shilpa Hiremath.; Chandraprabha, M. N.; Lourdu Antonyraj, M. A.; Indu Venu Gopal.; Aayushi Jain.; Kokil Bansal. Green synthesis of ZnO nanoparticles by Calotropis Gigantea. Int. J. Curr. Eng. Technol. 2013, 118-120. http://inpressco.com/category/ijcet.
- Ayesha Naveed Ul Haq.; Akhtar Nadhman.; Ikram Ullah.; Ghulam Mustafa.; Masoom Yasinzai.; Imran Khan. Synthesis Approaches of Zinc Oxide Nanoparticles: The Dilemma of Ecotoxicity. J. Nanomater. 2017.
- Happy Agarwal.; Venkat Kumar, S.; Rajesh Kumar, S. A review on green synthesis of Zinc oxide nanoparticles- An eco-friendly approach. Resource-Efficient Technologies. 2017. DOI:10.1016/j.reffit.2017.03.002.
- Yasser, A. Selim.; Maha, A. Azb.; Islam Ragab.; Mohamed, H.; Abd El-Azim, M. Green Synthesis of Zinc Oxide Nanoparticles Using Aqueous Extract of Deverra tortuosa and their Cytotoxic Activities. Sci. Rep. 2020, 10:3445.
- Khadeeja Parveen.; Viktoria Banse.; Lalita Ledwani. Green Synthesis of Nanoparticles: Their Advantages and Disadvantages. AIP Conference Proceedings 1724. 2016, 020048, DOI: 10.1063/1.4945168.
- Santhoshkumar, J.; Venkat Kumar, S.; Rajeshkumar, S. Synthesis of zinc oxide nanoparticles using plant extract against urinary tract infection pathogen. Resource-Efficient Technologies. 2017, 3, 459-465.
- Geetha, M.S.; Nagabhushana, H.; Shivananjaiah, H.N. Green mediated synthesis and characterization of ZnO nanoparticles using Euphorbia Jatropa latex as reducing agent, Journal of Science: Advanced Materials and Devices. 2016, 1, 301-310.
- Dayana Jeyaleela, G.; Rosaline Vimala, J.; Anthony Diwakar chandran, T.; Benno Susai Vijayakumar, A. Preliminary Phytochemical Analysis, Antioxidant Activity and Identification of Bioactive Compound in Stem and Leaf of Combretum Ovalifolium. Int. J. Sci. Technol. Res. 2020. 9, 3125-3132.
- Ravi Kant Sharma.; Ranjana Ghose. Synthesis of zinc oxide nanoparticles by homogeneous precipitation method and its application in antifungal activity against Candida albicans. Ceramics Int. 2014.
- Ming, W.Y.; Hua, L.J.; Yu, H.R. Large scale synthesis of ZnO nanoparticles via homogeneous precipitation. J. Cent. South. Univ. 2012, 19, 863–868.
- Joghee Suresh.; Ganeshan Pradheesh.; Vincent Alexramani.; Sun Ig Hong. Phytochemical Screening, Characterization and Antimicrobial, Anticancer Activity of Biosynthesized Zinc Oxide Nanoparticles Using Cyathea nilgiriensis Holttum Plant Extract. J. Bionanosci. 2018, 12, 1–12.
- Hamid Reza Ghorbani.; Ferdos Parsa Mehr.; Hossein Pazoki.; Behrad Mosavar Rahmani. Synthesis of ZnO Nanoparticles By Precipitation Method. Orient. J. Chem. 2015, 31, 1219-1221.
- Jafar Ahamed, A.; Vijaya Kumar, P. Synthesis and characterization of ZnO nanoparticles by co-precipitation method at room temperature. J. Chem. Pharm. Res. 2016, 8, 624-628.
- Julian Medina.; Harold Bolanos.; Lyda Patricia Mosquera‑Sanchez.; Rodriguez‑Paez, J. E. Controlled synthesis of ZnO nanoparticles and evaluation of their toxicity in Mus musculus mice. Int. Nano. Lett. 2018, 8,165–179.
- Vinoy Jacob.; Rajiv, P. In Vitro Analysis: The Antimicrobial And Antioxidant Activity Of Zinc Oxide Nanoparticles From Curcuma Longa. Asian. J. Pharm. Clin. Res. 2019, 12, 200-204.
- Siba Soren.; Sanjeet Kumar.; Sanjibani Mishra.; Padan, K. Jena.; Satish, K. Verma. Evaluation of antibacterial and antioxidant potential of the zinc oxide nanoparticles synthesized by aqueous and polyol method. Microb. Pathog. 2018, 119, 145–151.
- Huzaifa Umar.; Doga Kavaz.; Nahit Rizaner. Biosynthesis of zinc oxide nanoparticles using Albizia lebbeck stem bark, and evaluation of its antimicrobial, antioxidant, and cytotoxic activities on human breast cancer cell lines. Int. J. Nanomedicine, 2019, 14, 87–100.
- Adeleke, J.T.; Theivasanthi, T.; Thiruppathi, M.; Swaminathan, M.; Akomolafe, T.; Alabi, A.B. Photocatalytic degradation of methylene blue by ZnO/NiFe2O4 nanoparticles. Appl. Surf. Sci. 2018, 455, 195–200.
- Abebe Balcha.; Om Prakash Yadav.; Tania Dey. Photocatalytic degradation of methylene blue dye by zinc oxide nanoparticles obtained from precipitation and sol-gel methods. Environ. Sci. Pollut. Res. 2016. DOI 10.1007/s11356-016-7750-6.
- Dayana Jeyaleela, G.; Balasubramani, N.; Rosaline Vimala, J. Isolation and Characterization of Antioxidant (Flavone-3-Rutinoside, 3, 3’, 4’, 5, 7-Pentahydroxy) from Leaves of Melia Dubia. Asian. J. Pharm. Clin. Res.2019, 12, 107-114.
- Kavitha, S.; Dhamodaran, M.; Rajendra Prasad.; Ganesan, M. Synthesis and characterisation of zinc oxide nanoparticles using terpenoid fractions of Andrographis paniculata leaves. Int. Nano. Lett. 2017, 7, 141–147.
- Pragati Jamdagni.; Poonam Khatri.; Rana, J.S. Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. J. King Saud Univ. Sci.2016.
- Rajesh Kumar Shah.; Forishmeeta Boruah.; Nikahat Parween. Synthesis and Characterization of ZnO Nanoparticles using Leaf Extract of Camellia sinesis and Evaluation of their Antimicrobial Efficacy. Int. J. Curr. Microbio. Appl. Sci. 2015, 4, 444-450.
- Gunalan Sangeetha.; Sivaraj Rajeshwari.; Rajendran Venckatesh. Green synthesis of zinc oxide nanoparticles by aloe barbadensis miller leaf extract:Structure and optical properties. Mater. Res. Bull. 2011, 46, 2560–2566.
- Sadraei, R. A Simple Method for Preparation of Nano-sized ZnO. Res. Rev. J. Chem. 2016, 5, 45-49.
- Nurul Ain Samat.; Roslan Md Nor. Sol–gel synthesis of zinc oxide nanoparticles using Citrus aurantifolia extracts. Ceram. Int. 2013, 39, S545–S548.
- Pritam Sadhukhan.; Mousumi Kundu.; Sharmistha Chatterjee.; Noyel Ghosh.; Prasenjit Manna.; Joydeep Pritam, S. Das.; Parames, C. Sil. Targeted delivery of quercetin via pH-responsive zinc oxide nanoparticles for breast cancer therapy, Mater. Sci.Eng. C, 2019, 100, 129–140.
- Qiusen Han.; Xinhuan Wang.; Shuangfei Cai.; Xueliang Liu.; Yufei Zhang.; Lin Yang.; A Chen Wang.; Rong Yang. Quercetin nanoparticles with enhanced bioavailability as multifunctional agents toward amyloid induced neurotoxicity. J. Mater. Chem.B. 2018, 9.

This work is licensed under a Creative Commons Attribution 4.0 International License.









