A Simple, Green Approach towards Detecting Pollutant Gases using Flower Pigments from Anthurium andreanum
Rachelle Jenine D. Abuel and Voltaire G. Organo*
Department of Physical Sciences and Mathematics, College of Arts and Sciences, University of the Philippines, Manila, Philippines
Corresponding Author E-mail : vgorgano@up.edu.ph
DOI : http://dx.doi.org/10.13005/ojc/360427
Article Received on : 23 Apr 2020
Article Accepted on : 05 Jul 2020
Article Published : 16 Jul 2020
A simple and green approach towards detecting pollutant gases was developed using flower pigments derived from Anthurium andreanum. A drastic color change was observed when flower extracts, either in solution or adsorbed onto silica gel, were exposed to sulfur dioxide and nitrogen dioxide gas. Only a slight color change was observed for carbon dioxide. These results may be attributed to possible acid-base interaction and bisulfite addition or nitration to anthocyanin pigments in response to pollutant gases.
KEYWORDS:Anthurium andreanum Extracts; Colorimetric; Pollutant Gases; Flower Pigments
Download this article as:| Copy the following to cite this article: Abuel R. J. D, Organo V. G. A Simple, Green Approach towards Detecting Pollutant Gases using Flower Pigments from Anthurium andreanum. Orient J Chem 2020;36(4). |
| Copy the following to cite this URL: Abuel R. J. D, Organo V. G. A Simple, Green Approach towards Detecting Pollutant Gases using Flower Pigments from Anthurium andreanum. Orient J Chem 2020;36(4). Available from: https://bit.ly/2WruP4O |
Introduction
Pollutant gas may be described as any substance that would alter the chemical and biological properties of the atmosphere, causing injury or harm to the natural resources. Major gas pollutants include CO2, CO, SO2, NOx, volatile organic compounds (VOCs), and particulate matter.1 Increased amounts of these pollutant gases have led to health and environmental concerns.2,3 Hence, the identification and detection of pollutant gases remain to be the first step in monitoring and mitigating their effects.
Various gas-sensing technologies have been developed over time.4 One of the emerging solutions for detecting pollutant gases is through naked-eye detection. In the past decade, Suslick and co-workers5,6 developed colorimetric arrays using metalloporphyrin dyes, pH indicators, and solvatochromic dyes for the detection and identification of volatile organic compounds and other toxic gases. These detection methods were based on simple chemo-sensing principles, such as acid-base interactions, metal-ligand complexation, and polarity of analytes. A much simpler approach, however, makes use of pH indicators to detect specific gases. For example, Mills and co-workers7,8 developed a CO2-sensing ink and film based on m-cresol purple as an indicator.
A “greener” approach to gas detection is based on natural pigments. In 2014, Bernardo and Organo9 have demonstrated that chlorophyll extracted from leaves can be used to detect NO2 gas. The chlorophyll either in ethanolic solution or absorbed into filter paper changed color from green to yellow-brown upon exposure to NO2 gas. On the other hand, Solomon and co-workers10 used anthocyanin extracts from red cabbage as a sensing reagent for SO2 gas. The purple extract turned pink then colorless upon exposure to SO2 gas. This indicates the potential application of anthocyanin dyes for gas sensing.
Here, we demonstrate the possible use of natural pigments from flowers, specifically, Anthurium andreanum in detecting pollutant gases SO2, NO2, and CO2. The method presented here is simple, uses readily available reagents, and does not require expensive equipment for gas sensing.
Experimental
All reagents and chemicals used were analytical grade and purchased from commercial sources. Solvents were used without any purification.
Preparation of Pigments in Solution
Approximately 50 g of washed Anthurium andreanum petals were soaked in 250 mL reagent-grade ethanol. The mixture was stored inside the refrigerator for 24 hours. The filtrate was then collected and stored in amber bottles.
For gas sensing, 5 mL of crude extract was transferred to a 20-mL vial. 100 µL of 0.1M NaOH was then added to produce a purple-colored solution.
Preparation of Pigments in Silica Gel
3 g of silica powder was immersed in about 2 mL of the crude extract. The silica gel was air-dried under a fume hood until a blue colored powder was obtained. 0.2 g of silica powder samples were then transferred to four (4) separate 20-mL vials for gas exposure.
Exposure of Pigments to Analyte Gases
Production of gases was done in 20-mL vials and performed under the fume hood for safety purposes. SO2 and NO2 gases were generated based on procedures described by Solomon et. al. with slight modifications.10 Concentrated HCl (6M) was carefully added to 0.1 g of sodium bisulfite (NaHSO3) to produce colorless SO2. Likewise, amber-colored NO2 gas was produced upon the slow addition of concentrated HNO3 to a small piece of copper wire. CO2 gas was generated by carefully adding concentrated HCl (6M) to 0.1 g sodium bicarbonate (NaHCO3). Each gas was introduced directly to the flower extract in solution or silica gel using a syringe. Color changes were then noted.
Results and Discussion
Several studies have supported the use of flowers as natural indicators since their colors easily change with pH.11,12 Anthocyanins found in flower extracts are believed to be responsible for the color transformation. These compounds tend to undergo several structural forms with varied colors depending on the pH of the solution (Fig. 1).13 The red flavylium cation is the predominant form in very acidic solutions (pH < 3). The carbinol pseudo base is colorless but tends to rearrange into a yellow chalcone at pH 3-6. The formation of a purple neutral quinoidal base occurs at pH 6-8 while a blue anionic quinoidal base transpires at pH >8. The anthocyanins can thus serve as a sensor for water-soluble analytes via acid-base reactions14.
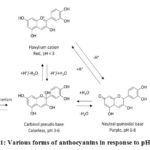 |
Figure 1: Various forms of anthocyanins in response to pH. |
Gas Sensing in Solution
In this study, anthocyanins found in flower extracts of Anthurium andreanum14,15 were investigated for its potential use as an indicator for pollutant gases SO2, NO2, and CO2. The flower extracts were observed to change color instantaneously upon exposure to gases, as shown in Fig.2.
 |
Figure 2: Ethanolic flower extracts upon exposure to SO2, NO2, and CO2. |
Exposure of the crude extract to SO2 led to a color change from purple to bright red. This qualitative observation suggests that the pigments shift to their acidic color. SO2 gas may have reacted with small amounts of water in the ethanolic solution to produce H2SO3, as explained by Solomon.10
SO2 (g) + H2O![]() H2SO3 (aq)
H2SO3 (aq) ![]() H+(aq) + HSO3–(aq)
H+(aq) + HSO3–(aq)
At the onset of lowering the pH, the protonated monomeric form (flavylium cation) of the pigments in Anthurium predominates, thus, exhibiting a bright red coloration.13
Similarly, exposure of the crude extract to NO2 led to a shift in the pigment’s acidic color (bright red). NO2 may also have reacted with water, according to the reaction below, to produce acidic species.10 This may cause a decrease in the pH of the solution and the corresponding color change.
2NO2(g) + H2O![]() 2H+ (aq) + NO3– (aq) + NO2– (aq)
2H+ (aq) + NO3– (aq) + NO2– (aq)
On the other hand, exposure of the extract to CO2 does not exhibit a prominent color change in the solution. Even though CO2 can react with water, the carbonic acid formed is weaker (pKa1 = 6.35) compared to sulfurous acid (pKa1= 1.89) and nitrous acid (pKa= 3.29).16 As a result, the amount of hydronium ions produced in solution is not sufficient enough to shift the equilibrium of anthocyanin towards the flavylium form.
Gas Sensing in Silica Gel
While color changes were observed at the onset, the pigments were found to be unstable in solution, as observed by further color change through time. It was, therefore, necessary to stabilize the color of the flower extract for the duration of the study. Silica gel proved to be an excellent solid medium for this purpose. Mixing the extract with silica gel produced a blue powder on drying. This powder was then exposed to gases, resulting in varied colors depending on the gas (Fig. 3).
 |
Figure 3: Flower extracts in silica gel upon exposure to SO2, NO2, and CO2. |
For SO2 gas, the silica gel turned pink instantly. This color change was not observed in solution under the same conditions. However, Solomon and co-workers10 also observed a similar pink color when anthocyanin from red cabbage was exposed to SO2 gas. The color may be attributed to the formation of the flavylium cation at low pH which was then decolorized by SO2. It has been proposed in previous studies that the bisulfite ion formed may undergo a reversible addition to the aglycone core of the anthocyanin, forming a colorless bisulfite adduct (Fig. 4).10,17
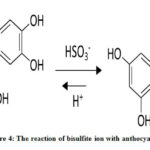 |
Figure 4: The reaction of bisulfite ion with anthocyanin. |
A yellow-colored powder is obtained upon the reaction of NO2 with the pigments in silica gel. Unlike in solution, NO2 may have reacted differently with the flower pigments in silica gel. Nitration of the aromatic moieties of the pigments may have taken place other than the acid-base reaction. Studies on the nitration of natural pigments to form yellow-colored products have been reported in literature.9,18,19
On the contrary, exposing the pigment with CO2 using silica gel slightly changes the blue-colored pigment to a purple hue. The color change, however, was not very apparent. This observation may be attributed to a weak acid-base interaction with the flower pigments, similar to that in solution.
Conclusion
In conclusion, a simple and cheap colorimetric indicator for pollutant gases based on natural pigments was developed. The pigments can be obtained easily from the flowers of Anthurium andreanum, and can be used either in solution or adsorbed onto silica gel. The flower extracts were found to be responsive towards SO2 and NO2 gases. Only slight color changes were observed for CO2. Thus, this method presents a “green” approach towards the development of “naked-eye” detection of SO2 and NO2 gases. It also demonstrates the destructive effect of these pollutant gases on flower pigments. Further work will be directed towards improving the detection limit and applying it as a sensor for environmental monitoring.
Acknowledgment
Financial support was provided by the Creative Work and Research Grant (CWRG Grant No. 2012-25) of the University of the Philippines System.
Conflict of Interest
The authors do not have any conflict of interest.
Funding Source
Financial support was provided by the Creative Work and Research Grant (CWRG Grant No. 2012-25) of the University of the Philippines System.
References
- Kibble, A.; Harrison, R. Point Sources of Air Pollution. Med. 2005, 55, 425–431.
CrossRef - Reames, T. G.; Bravo, M. A. People, Place and Pollution: Investigating Relationships between Air Quality Perceptions, Health Concerns, Exposure, and Individual- and Area-Level Characteristics. Int. 2019, 122, 244–255.
CrossRef - Bessac, B. F.; Jordt, S. E. Sensory Detection and Responses to Toxic Gases: Mechanisms, Health Effects, and Countermeasures. Am. Thorac. Soc. 2010, 7, 269-277.
CrossRef - Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A Survey on Gas Sensing Technology. Sensors 2012, 12 (7), 9635–9665.
CrossRef - Janzen, M. C.; Ponder, J. B.; Bailey, D. P.; Ingison, C. K.; Suslick, K. S. Colorimetric Sensor Arrays for Volatile Organic Compounds. Chem. 2006, 78, 3591-3600.
CrossRef - Feng, L.; Musto, C. J.; Kemling, J. W.; Lim, S. H.; Zhong, W.; Suslick, K. S. Colorimetric Sensor Array for Determination and Identification of Toxic Industrial Chemicals. Chem. 2010, 82 (22), 9433–9440.
CrossRef - Mills, A.; Skinner, G. A.; Grosshans, P. Intelligent Pigments and Plastics for CO2 J. Mater. Chem. 2010, 20, 5008–5010.
CrossRef - Mills, A.; Skinner, G. A. Water-Based Colourimetric Optical Indicators for the Detection of Carbon Dioxide. Analyst 2010, 135, 1912–1917.
CrossRef - Bernardo, M. K. O.; Organo, V. G. Chlorophyll as a Simple, Inexpensive and Environment-Friendly Colorimetric Indicator for NO2 Orient. J. Chem. 2014, 30 (2), 445–449 .
CrossRef - Solomon, S.; Oliver-Hoyo, M.; Hur, C. Generating Water-Soluble Noxious Gases: An Overhead Projector Demonstration. Chem. Educ. 1998, 75 (12), 1581–1582 .
CrossRef - Mohd, P.; Khan, A.; Farooqui, M. Analytical Applications of Plant Extract as Natural pH Indicator : A Review. Adv. Sci. Res. 2011, 2 (4), 20–27.
- Gupta, P.; Jain, P.; Kumar Jain, P. Isolation of Natural Acid-Base Indicator from the Flower Sap of Hibiscus rosa sinensis. Chem. Pharm. Res. 2012, 4 (12), 4957–4960.
- Castaneda-Ovando, A.; Pacheco-Hernandez, M. de L.; Peez-Hernandez, M. E.; Rodriguez, J.; Galan-Vidal, C. A. Chemical Studies of Anthocyanins: A Review. Food Chem. 2009, 113 (4), 859–871.
CrossRef - Galingana, M. O.; Organo, V. G. A Simple Colorimetric Procedure for Differentiating Anions Using Flower Pigments from Anthurium andreanum. J. Chem. 2016, 32 (3), 1347–1352.
CrossRef - Li, C.; Yang, G.; Huang, S.; Lü, D.; Wang, C.; Chen, J.; Yin, J. Characterisation of Flavonoids in Anthurium Spathes and Their Contribution to Spathe Colouration. Hortic. Sci. Biotechnol. 2013, 88 (2), 208–215.
CrossRef - Perrin, D. D. Dissociation Constants of Inorganic Acids and Bases in Aqueous Solution. Pure Appl. Chem. 1969, 20, 133–236.
CrossRef - He, F.; Liang, N. N.; Mu, L.; Pan, Q. H.; Wang, J.; Reeves, M. J.; Duan, C. Q. Anthocyanins and Their Variation in Red Wines I. Monomeric Anthocyanins and Their Color Expression. Molecules. 2012, 17, 1571–1601.
CrossRef - Fanning, J. C.; Mandel, F. S.; Gray, T. L.; Datta-Gupta, N. The Reaction of Metalloporphyrins with Nitrogen Dioxide. Tetrahedron 1979, 35 (10), 1251–1255.
CrossRef - Catalano, M. M.; Crossley, M. J.; Harding, M. M.; King, L. G. Control of Reactivity at the Porphyrin Periphery by Metal Ion Co-Ordination: A General Method for Specific Nitration at the b-Pyrrolic Position of 5,10,15,20-Tetraarylporphyrins. J. Chem. Soc., Chem. Commun.1984, 1535–1536.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









