A Comparative Study on Biological Activity of Schiff Bases Derived from 2-(2-amino)-3-(1H-indol-3-yl)propanoic Acid
Santhi Radhakrishnan1*, Rajeswari Balakrishnan1,2 and Ahalya Selvaraj1
1Department of Chemistry, Seethalakshmi Ramaswami College, Affiliated to Bharathidasan University, Tiruchirappalli – 620 002, TamilNadu, India.
2K. Ramakrishnan College of Engineering, Kariyamanikam Road, Samayapuram, Tiruchirappalli – 621 112, TamilNadu, India.
Corresponding Author E-mail : santhichemsrc@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/360426
Article Received on : 10 May 2020
Article Accepted on : 15 July 2020
Article Published : 15 Jul 2020
Indole group containing four different Schiff bases such as 2-((2-hydroxybenzylidene)amino)-3-(1H-indol-3-yl)propanoic acid, 2,2'-((1Z,1'E)-(1,4-phenylenebis(methanylylidene))bis(azanylylidene))bis(3-(3a,7a-dihydro-1H-indol-3-yl)propanoic acid), (E)-2-((4-chlorobenzylidene)amino)-3-(3a,7a-dihydro-1H-indol-3-yl)propanoic acid were synthesized from Tryptophan and respective aldehydes namely Salicylaldehyde, Terephthalaldehyde, 2-Chlorobenzaldehyde and 4-Chlorobenzaldehyde. A polymeric composite was prepared by doping the Schiff base derived from salicylaldehyde on polyaniline chloride. These compounds were characterized by Fourier –transform infrared (FTIR), UV-Visible, 1H- NMR spectroscopic techniques. The Schiff Bases and polymer have been examined for their antimicrobial activity. Antibacterial and antifungal activities of all the compounds were studied by Kirby Bauer Agar well diffusion method. In addition Anti-Tubercular study was also performed. It has been found that all the five compounds have remarkable antimicrobial activity.
KEYWORDS:Chlorobenzaldehyde; Polymer and Antimicrobial; Schiff Base; Tryptophan
Download this article as:| Copy the following to cite this article: Radhakrishnan S, Balakrishnan R, Selvaraj A. A Comparative Study on Biological Activity of Schiff Bases Derived from 2-(2-amino)-3-(1H-indol-3-yl)propanoic Acid. Orient J Chem 2020;36(4). |
| Copy the following to cite this URL: Radhakrishnan S, Balakrishnan R, Selvaraj A. A Comparative Study on Biological Activity of Schiff Bases Derived from 2-(2-amino)-3-(1H-indol-3-yl)propanoic Acid. Orient J Chem 2020;36(4). Available from: https://bit.ly/32lWHv2 |
Introduction
The preparation of imines was first reported in the 19th century by Hugo Schiff (1864). In the recent past years various methods including solvent free synthesis and microwave irradiation have been reported for the synthesis of Schiff bases.1 Schiff bases have photochromic and thermochromic properties that made it to be used in modern technologies such as optical computers, measuring the intensity of radiation, imaging system and in molecular memory storage.2,3 These imines are found to have various applications in food industry, dye industry, agrochemical industry and catalysis. In Chemistry, Schiff bases are used in quantitative analysis as potentiometric sensors, solvent extraction reagents and solid phase extraction sorbent.4 In addition to these applications right from 19th century in the field of antimicrobial drugs, the imines are found to play a vital role. Schiff Base compounds are key part of coordination chemistry as they lead to the formation of stable complexes.5 Schiff bases are said to have antibacterial,6 antiviral,7 antifungal,8 anticancer,9 antitubercular,10 antimalarial,11 analgesic,12,13 anti-inflammatory,14 anticonvulsant15 and antioxidant16 properties. Tryptophan is a non-polar aromatic amino acid which is less explored in the field of antimicrobial activity. Tuberculosis is an airborne respiratory disease and in India there are more than 1 million cases is being reported per year. In the present investigation, the aim is to synthesise some new Schiff bases from tryptophan and to study antibacterial activity against certain pathogens such as Bacillus subtilis, Enterococcus, Proteus vulgaris, Staphylococcus aureus, Klebsiella pneumonia, Pseudomonas Aeruginosa, Salmonella typhi, Escherichia Coli, antifungal activity against Candida albicans, Trichoderma, Aspergillus niger and antitubercular actitvity using Mycobacterium tuberclosis. An attempt is also made to understand antimicrobial activities of a polymer by doping 2-((2-hydroxybenzylidene)amino)-3-(1H-indol-3-yl)propanoic acid on polyaniline chloride and to compare with that of Schiff bases.
Materials and Methods
Materials
Commercially available AR-grade chemicals and solvents were purchased and used as such. A Buchi melting point apparatus was used to determine the melting points of the Schiff base compounds. UV-Visible Spectrophotometer (Perkin Elmer Lambda 35) was used to record UV-Visible spectrum. FTIR spectral measurements were carried out using Perkin Elmer FTIR spectrophotometer. H1-NMR spectra were recorded on Bruker 400 NMR spectrometer using TMS as standard.
Methods
Synthesis of Schiff Bases
The azomethine compounds were synthesized by condensing Tryptophan and corresponding carbonyl compounds in the ratio 1:1 except terephthalaldehyde (2:1). Tryptophan and aldehydes were ground well in presence of small amount of acetic acid. The resulting mixture was refluxed for 4-6 hours. The product formed was filtered, washed with ethanol and dried using anhydrous CaCl2 in a vacuum desiccator. The samples were recrystalised with ethanol.
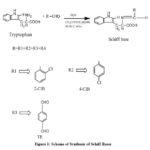 |
Figure 1: Scheme of Synthesis of Schiff Bases |
Preparation of Poly Aniline Chloride
Aniline (0.1M) was dissolved in aqueous solution of hydrochloric acid (1M). The mixture was stirred using magnetic stirrer and an aqueous potassium persulphate solution (0.1M) was added drop wise to the aniline-acid mixture for about two hours with continuous stirring at room temperature. After the addition of potassium persulphate the stirring was continued to ensure complete polymerisation. The PANI formed was green in colour. It was filtered using Whatmann No:1 filter paper and washed with distilled water many times to remove excess acid content and oligomers of aniline followed by ethanol, acetone and diethyl ether. The polymer was dried in an oven at about 373 K for four hours till constant weight was reached.17
The colour changes that appeared during the addition of potassium persulphate is given below
Straw yellow → Yellow → Brown → Green colour
 |
Figure 2: Structure of PANI chloride |
Preparation of Polyaniline Chloride – Schiff Base Composite
Polyaniline chloride (0.1g) and Schiff base (0.1g) were mixed together in 25ml of diethyl ether and sonicated for one hour.16 The resulting slurry was washed with ether and centrifuged. The resulting green coloured mass was dried in a vacuum desiccator to get a free flowing powder.
Biological Activity
The Schiff bases (2-((2-hydroxybenzylidene)amino)-3-(1H-indol-3-yl)propanoic acid, 2,2′-((1Z,1’E)-(1,4-phenylenebis(methanylylidene))bis(azanylylidene))bis(3-(3a,7a-dihydro-1H-indol-3-yl)propanoic acid), (E)-2-((2-chlorobenzylidene)amino)-3-(3a,7a-dihydro-1H-indol-3-yl)propanoic acid and (E)-2-((4s-chlorobenzylidene)amino)-3-(3a,7a-dihydro-1H-indol-3-yl)propanoic acid) and the polymeric composite were screened for antibacterial, antifungal and antitubercular activity by well method. Bacterial cultures such as Bacillus subtilis, staphylococcus aureus, Enterococcus, proteus vulgaris, salmonella typhi, klebseilla puminia, Escherichia Coli, Pseudomonas Aeruginosa were used for understanding antibacterial activity and candida albicans, A.niger & Trichoderma have been applied to study antifungal activity. Anti tubercular activity was evaluated using mycobacterium tuberculosis. Nutrient agar medium was used for culture of bacteria. 3.0 g of beef- extract, 5.0g of sodium chloride, 15.0g of agar-agar were added into 1000ml of distilled water. Then this medium was autoclaved at 15 psi & 394K for 15 minutes, then the medium was allowed to solidify by pouring into the sterile petriplates. A sterile bud was used to swab the bacterial broth culture on each petriplates. Five wells (4 wells in periphery and 1 in centre) were made in the medium and subsequently peripheral wells were filled with 25,50,75,100 ppm solutions of synthesized compound respectively and the central well was filled with gentamicin at the same concentration. Other petridishes were sealed with paraffin & incubated at 310-311K for bacterial strain and 298K (fungi) by following the general procedure. Gentamycin has been employed as a control. After incubation the plates have been examined for the zone of inhibition(ZOI) and compared with that of the standard one.
Results and Discussion
The imines and polymeric composite have been synthesized using reported procedure. The yield was good and all compounds were found to be stable. The physical properties of the synthesized compounds are mentioned in table 1.
IR Spectra
In the FTIR spectra, the appearance of azomethine -HC=N band in the range 1651 – 1664 cm-1 confirms the formation of Schiff bases (TRSA, TPTE, TP2ClB, TP4ClB). The bending vibrations of the various groups are found in the region 400-960 cm-1. The bending vibrations below 600 cm-1 corresponding to OH & Cl present in the carbonyl moiety. The bands at 1436-1516cm-1 are due to C=C of aromatic ring. The band in the region of 3003-3060 cm-1 may be assigned to aromatic and aliphatic C-H groups.18 The bands at 3388-3427 cm-1 are ascertained to NH group of the tryptophan moiety (Fig 3a-3d). FT-IR spectra of PANI composite (Fig.3e) shows a band in the region 3233cm-1 which are due to N-H, C-H and O-H stretching vibrations in the polymer. Appearance of bands in the region 600-800cm-1 are due to p-disubstituted aromatic ring in the PANI backbone. A peak at 1501cm-1 may be due to -C=C- present in aromatic ring. The stretching frequency at 1588cm-1 has been assigned to the C-C vibrations in benzenoid and quinoid forms. A characteristic band at 1618cm-1 may be due to -HC=N stretching mode of Schiff base doped on polyaniline.
Electronic Spectra
The electronic spectra of Schiff base compounds were recorded using DMSO as solvent between 200-700nm at room temperature. Two bands are observed in the region of 296-308nm corresponding to π-π* transition of phenyl rings in tryptophan molecule. A broad band at 321-360nm may be due to n-π* transition of azomethine -HC=N chromophore. It is well known that Schiff bases exhibit two peaks for π-π* transition which are very sharp at lower wavelength region, while n-π* band occurring between higher wavelength region is a broad band19,20 (Fig 4a-4d). In the electronic spectra of polymeric composite, a band at 320nm may be attributed to the presence of -HC=N group and a band at about 622nm indicate that the composite contain PANI skeleton (Fig 4e).
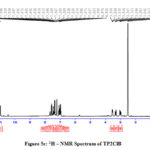
1H NMR Spectra
The 1H NMR spectra of the Schiff bases were recorded in DMSO. The peaks due to azomethine proton appear at 7.5 -8.1 ppm for all the prepared imines.21 The signals at 6.7 – 7.5 ppm can be ascribed to Schiff base aromatic protons. A peak at 10.9ppm corresponding to the acidic proton of tryptophan moiety is observed for all the compounds. In TPSA a small peak appears at 4.3ppm due to the presence of phenolic proton of salicylaldehyde (Fig 5a-5d).
The 1H-NMR spectra (Fig.5e) of the composite shows signal at δ10.2-10.8ppm due to the –OH proton of carboxyl group present in dopant. The signal at 8.2 ppm are due to imine protons in Schiff base. The signals at δ7.0-7.6 ppm are due to PANI aromatic –CH and –NH protons. The peaks at δ6.8-6.9 ppm may be attributed to quinoid CH protons.
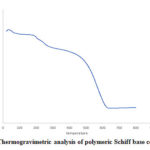
Thermo Gravimetric Analysis
TGA thermogram recorded for PANI chloride-Schiff base composite is presented in Fig.6. It shows that the polymeric composite is stable upto 6000C as the compound exhibits a linear decline upto 6000C, after which there is no weight loss.
Table 1: Physical data of the compounds
|
S. No |
Entry |
IUPAC Name |
Molecular Formula |
Molecular Weight |
Colour |
Melting point |
|
1 |
TRSA |
2-((2-hydroxybenzylidene)amino)-3-(1H-indol-3-yl)propanoic acid |
C18H17N2O3 |
309.34 |
Yellow |
196oc |
|
2 |
TPTE |
2,2′-((1Z,1’E)-(1,4-phenylenebis(methanylylidene))bis(azanylylidene))bis(3-(3a,7a-dihydro-1H-indol-3-yl)propanoic acid) |
C19H17N2O3 |
321.35 |
White |
130 oc |
|
3 |
TP2ClB |
(E)-2-((2-chlorobenzylidene)amino)-3-(3a,7a-dihydro-1H-indol-3-yl)propanoic acid |
C18H16N2O2Cl |
327.78 |
Sandal |
173 oc |
|
4 |
TP4ClB |
(E)-2-((4-chlorobenzylidene)amino)-3-(3a,7a-dihydro-1H-indol-3-yl)propanoic acid |
C18H16N2O2Cl |
327.78s |
Pale Yellow |
230 oc |
 |
Figure 3a: IR spectrum of TPSA |
 |
Figure 3b: IR spectrum of TPTE |
 |
Figure 3c: IR spectrum of TP2ClB |
 |
Figure 3d: IR spectrum of TP4ClB |
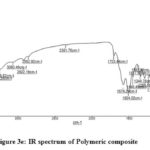 |
Figure 3e: IR spectrum of Polymeric composite |
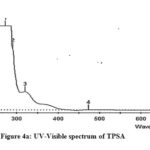 |
Figure 4a: UV-Visible spectrum of TPSA |
 |
Figure 4b: UV-Visible spectrum of TPTE |
 |
Figure 4c: UV-Visible spectrum of TP2ClB |
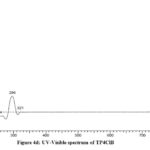 |
Figure 4d: UV-Visible spectrum of TP4ClB |
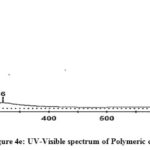 |
Figure 4e: UV-Visible spectrum of Polymeric composite |
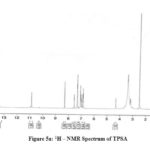 |
Figure 5a: 1H – NMR Spectrum of TPSA |
 |
Figure 5b: 1H – NMR Spectrum of TPTE |
|
|
Figure 5c: 1H – NMR Spectrum of TP2ClB |
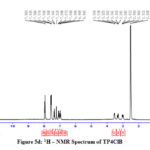 |
Figure 5d: 1H – NMR Spectrum of TP4ClB |
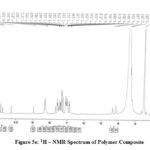 |
Figure 5e: 1H – NMR Spectrum of Polymer Composite |
|
|
Figure 6: Thermogravimetric analysis of polymeric Schiff base composite |
Antimicrobial Activity
The shortage of new antimicrobial drugs and resistance to antimicrobial agents kindled the authors to study antibacterial, antifungal and antitubercular activity using gram positive bacteria (Staphylococcus aureus, Bacillus subtilis & Enterococcus), gram negative bacteria (Proteus vulgaris, Salmonella typhi, Klebsiella pneumonia & Escherichia Coli), fungai( candida albicans, Aspergillus niger, Trichoderma) and mycobacterium tuberculosis respectively. The maximum zone of inhibition for all the prepared compounds were determined by well method. The antimicrobial activities of all the compounds including a composite are found to increase with increase in concentration of experimental solutions (Table 2). The compound TRSA shows greater value of ZOI than the control at 100µl against all the tested micro organisms while other Schiff base compound exhibit either lesser value or equal value as that of control. Thus all the chosen compounds show significant antimicrobial activity against all the strains due to the presence of azomethine moiety in all the compounds. The difference in activity may be attributed to difference in structure of molecules.
When the results at same concentrations are compared between Schiff base monomers and the composite it is revealed that the polymer is more effective than its dopant. This may be attributed to the presence of long conjugated chain present in polymeric back bone.22 Polymeric antimicrobial agents have advantage over the monomers due to the fact that they are chemically stable and are not permeable through skin.
A perusal of biological study indicates that the compound TP4ClB is more effective than that of TP2ClB. This may be due to the difference in positions of -Cl group in both the compounds. In TP4ClB, -Cl is present in para position leading to more conjugation. According to the present study the polymeric composite can act as a good antibacterial and antifungal agents while the compound TRSA can be suggested as a better antitubercular agent in comparison with other prepared compounds (Fig 7).
Table 2: Antimicrobial Activity
|
S.No |
Name of the microorganisms |
Zone of inhibition in mm/µl |
||||||||||||||||||||||||
|
TRSA |
TPTE |
TP2ClB |
TP4ClB |
Composite |
||||||||||||||||||||||
|
25 µl |
50 µl |
75 µl |
100 µl |
Control |
25 µl |
50 µl |
75 µl |
100 µl |
Control |
25 µl |
50 µl |
75 µl |
100 µl |
Control |
25 µl |
50 µl |
75 µl |
100 µl |
Control |
25 µl |
50 µl |
75 µl |
100 µl |
Control |
||
|
1 |
Bacillus subtilis |
16 |
18 |
22 |
25 |
22 |
10 |
12 |
14 |
16 |
20 |
– |
– |
10 |
12 |
20 |
14 |
17 |
21 |
26 |
20 |
20 |
22 |
24 |
28 |
20 |
|
2 |
Staphylococcus aureus |
16 |
18 |
20 |
25 |
22 |
– |
– |
10 |
12 |
26 |
– |
– |
10 |
12 |
26 |
10 |
12 |
15 |
17 |
18 |
20 |
23 |
27 |
30 |
20 |
|
3 |
Enterococcus |
– |
10 |
12 |
14 |
16 |
15 |
– |
10 |
12 |
14 |
15 |
10 |
12 |
14 |
16 |
20 |
– |
||||||||
|
4 |
Proteus vulgaris |
– |
10 |
13 |
15 |
18 |
15 |
– |
10 |
12 |
14 |
15 |
10 |
12 |
14 |
16 |
16 |
– |
||||||||
|
5 |
Klebsiella pneumonia |
– |
10 |
12 |
14 |
16 |
15 |
– |
10 |
12 |
14 |
30 |
12 |
14 |
16 |
18 |
18 |
|
||||||||
|
6 |
Salmonella typhi |
– |
12 |
16 |
20 |
24 |
25 |
– |
10 |
12 |
14 |
20 |
12 |
13 |
14 |
15 |
18 |
– |
||||||||
|
7 |
Escherichia Coli |
15 |
17 |
20 |
24 |
20 |
– |
– |
– |
20 |
24 |
29 |
34 |
20 |
||||||||||||
|
8 |
Pseudomonas Aeruginosa |
16 |
19 |
23 |
26 |
23 |
– |
– |
– |
20 |
23 |
27 |
30 |
25 |
||||||||||||
|
9 |
Candida albicans |
16 |
18 |
22 |
24 |
25 |
14 |
16 |
18 |
20 |
25 |
– |
10 |
12 |
14 |
22 |
14 |
16 |
18 |
20 |
20 |
18 |
20 |
23 |
26 |
24 |
|
10 |
Aspergillus niger |
14 |
17 |
20 |
23 |
23 |
– |
– |
12 |
14 |
25 |
10 |
12 |
14 |
16 |
25 |
10 |
12 |
14 |
16 |
18 |
18 |
20 |
24 |
26 |
20 |
|
11 |
Trichoderma |
20 |
25 |
30 |
30 |
20 |
– |
– |
– |
16 |
19 |
24 |
28 |
22 |
||||||||||||
 |
Figure 7: Antimicrobial activity of Schiff Bases and Polymer (mm/100µl) |
Conclusion
Tryptophan is an essential chemical compound that helps the body to form proteins and certain brain signaling species. The synthesis of Schiff bases from tryptophan and a polymeric composite by doping one of the imines on poly aniline chloride and characterization by spectral and analytical methods have been reported in the present paper. All the prepared imines and polymer have been evaluated for their anti microbial activity and found to have significant anti bacterial, anti fungal and anti tubercular activity against tested microorganisms. It can be concluded that all the investigated compounds may be used in various applications as anti microbial agents.
Acknowledgements
The authors acknowledge the management of Seethalakshmi Ramaswami College, Tiruchirappalli – 620 002 for the facilities provided to carry out the reported work. The authors express their sincere thanks to SASTRA Deemed University, Thanjavur for NMR Study and St. Joseph College, Tiruchirappalli for UV-visible and FT-IR studies.
Conflict of Interest
The authors declared that there is no conflict of interest regarding the publication of this paper.
References
- Cleiton M.da Silva; Angelo de Fatima. Adv. Res. 2011, 2, 1-8.
- Tanaka, A.K.; Shimoura, R.; Caira, M. R. Tetrahedron Lett. 2010, 2, 449-452.
CrossRef - Marisol Ibarra-Rodriguez; Munoz, B.M.; Rasika Dias, H.V. Org. Chem. 2017, 5, 2375-2385.
CrossRef - Nabil Ramadan Bader. Rasayan J. Chem. 2010, 3, 660-670.
- Clarke, B.; Clarke, N.; Cunningham, D.; Higgins T.; McArdle P.Ni Cholchu; O’Gara M. Organomet. Chem. 1998, 559, 55-64.
CrossRef - Kelode, S.R.; Mandlik, P.R.; Aswar, A.S. J. Chem. 2011, 27, 1053-1062.
CrossRef - Krishnan Suresh Kumar; Swastika Ganguly. J. Med. Chem. 2010, 45, 5474-5479.
CrossRef - Sachin R. Joshi; Seema I. Habib. J. Chem. 2014, 30, 1343-1348.
CrossRef - Mokhles M.Abd-Elzaher; Ahmed A.El-Rashedy. Beni-Suef University Journal of Basic and Applied Sciences 2016, 5, 85-96.
CrossRef - Ganesh More; Sakina Bootwala; J. Chem. 2018, 34, 800-812.
CrossRef - Scott E Harpstrite; Silvia D Collins. Chem. 2008, 4, 392-395.
CrossRef - Chinnasamy, R.P.; Sundararajan, R.; Govindaraj, S. Adv.Pharm.Technol Res. 2010, 1, 342.
CrossRef - Pandey, A.; Dewangan, D.; Verma, S.; Mishra, A., Dubey, R.D. J.Chem.Tech.Res. 2011, 3, 178-184
- Sathe, B.S.; Jayachandran, E.; Jagtap, V.A.; Sreenivasa, G.M. J.Phar.Res. 2011, 3, 164-169.
- Chaubey, A.K.; Pandeya, S.N. J.Pharm.Technol.Res. 2012, 4, 590-598.
- Wei, D.; Li, N.; Lu, G.; Yao, K. China B Chem. Life Sci. Earth Sci. 2006, 49, 225-229.
CrossRef - Rajeswari, B.; Santhi, R.; Radha, N. Indian J. Appl. Res. 2019, 9, 1-5.
- Adem Cinarli; Demet Gurbuz; Aydin Javman; Seher Birteksoz A. Chem.Soc.Ethiop. 2011, 25, 407-417.
CrossRef - Silverstein, R.M., Bassler, G.C., Morril, T.C., Spectrometric Identification of organic compounds, 5th ed., 1991, 110-111.
- Mohamed Mustafa; Hapipah, I.; Mahmood Ameen Abdulla; Robinson Ward T. 2009, 28, 3993-3998.
CrossRef - Salunke M.H.; Filmwala Z.A.; Dharap S.B.; Kamble A.D. J. Chem. 2011, 27, 1185-1191
- Moina Akhtar Mughal; Akhtar Hussain Mughal; Zeenat M.Ali; Ghulam Zuhra Memon; Mohammad Yar Khuhawar; Hussains Saleem. IOSRJEN. 2013, 3, 48-55.

This work is licensed under a Creative Commons Attribution 4.0 International License.