Synthesis and Physico-Chemical Studies of Ni(II) and Zn(II) Complexes with Schiff’s Bases of Thiophene-2-Thiohydrazide
1Department of Chemistry, L.S.College, B.R.A.B.U, Muzfffarpur, Bihar, India.
2Department of Chemistry, Motihari Engineering College, Motihari, Bihar, India
3Department of Chemistry, R.B.B.M.College, B.R.A.B.U, Muzaffarpur, Bihar, India
Corresponding Author E-mail: karbindus@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/360333
Article Received on : 18 Mar 2020
Article Accepted on : 12 Jun 2020
Article Published : 09 Jun 2020
The complexes of Nickel(II) and Zinc(II) with Schiff’s bases of thiophene-2-thiohydrazide of compositions [M(LH)2] [M=Ni2+ or Zn2+ and LH=Acetone-thiophene-2-thiohydrazone (Hactth), Acetylacetone-thiophene-2-thiohydrazone (Hacactth), Benzaldehyde-thiophene-2-thio-hydrazone (Hbentth), Furfural thiophene-2-thiohydrazone(Hffth)]; [ML2], [ML(H2O)].nH2O (n=0 or 2); [ML(NH3)] and [ML(Py)]; [M=Ni2+ or Zn2+ and LH2=Salicylaldehyde-thiophene-2-thiohydrazone (H2Saltth), 2-hydroxyacetophenone thiophene-2-thiohydrazone (H2aptth)] have been prepared and characterized by elemental analysis, spectral (UV-UIS, IR, 1HNMR) Studies, electrical conductance values, thermogravimetric analysis and magnetic Susceptibility measurements at room temperature. Some of the ligands as well as their Ni(II) and Zn(II)complexes exhibit appreciable antifungal activity.
KEYWORDS:Antifungal Activity; Schiff’s Base; Spectral Studies; Thiohydrazones; Thermogravimetric Analysis
Download this article as:| Copy the following to cite this article: Kumar A, Sinha P. K, Roy D. P. Synthesis and Physico-Chemical Studies of Ni(II) and Zn(II) Complexes with Schiff’s Bases of Thiophene-2-Thiohydrazide. Orient J Chem 2020;36(3). |
| Copy the following to cite this URL: Kumar A, Sinha P. K, Roy D. P. Synthesis and Physico-Chemical Studies of Ni(II) and Zn(II) Complexes with Schiff’s Bases of Thiophene-2-Thiohydrazide. Orient J Chem 2020;36(3). Available from: https://bit.ly/30p7iEs |
Introduction
Thiohydrazides and thiohydrazones are one of the most Studied ligands owing to their flexibility of binding sites to metal ions, broad range of biological activity and various commercial applications like, wear inhibiting additives in lubricants, vulcanization accelerators for rubber, specific analytical reagents etc.1-6 We have already reported the complexes of a number of metal ions with various thiohydrazides and their Schiff’s bases.7-8 Many other workers9-10 have also synthesised and characterized the complexes of these ligands using different metals, but little work has been carried out on the complexes of Schiff’s bases of bioiogically active thiophene-2-thiohydrazide. The capability of thiohydrazones to coordinate to metal ions is due to the delocalization of electron density over the chelate ring. Keeping in view, ‘2+’ as the most prolific oxidation state of Nickel & Zinc which is also easily accessible for complex formation, very alluring stereochemistry of Ni(II) complexes, involvement of both these elements in several biological processes and immense utility of their complexes,11-14 we report here the preparation, characterization, thermal and biological studies of complexes of Ni(II) & Zn(II) with thiophene-2-thiohydrazones derived from acetone (Hactth), acetylacetone (Hacactth); benzaldehyde(Hbentth), Salicylaldehyde (H2Saltth); 2-hydroxyacetophenone (H2aptth) and furural (Hftth).
Experimental
All the reagents were of E.Merck (AR arade) and the solvents were purified by standard methods and dried before use. The magnetic susceptibility was determined at room temperature by Gouy method. IR Spectra were recorded as KBr Pellets or nujol mull in the range 400-4000 cm-1 on Perkin. Elmer FTIR-Spectrometer. The electronic absorption Spectra of the complexes and ligands were recorded in the range of 220-900 nm in ethanol, dioxane or CCl4 at RSIC Chandigarh, CDRI-Lucknow as well as in the P.G.Deptt. of Chemistry, B.R.A.B.U. Muzaffarpur. 1HNMR Spectra were recorded from CDRI. Lucknow. Sulphur was estimated gravimetrically as BaSO4. The metal ions were estimated by standard methods.15 Thiophene-2-thiohydrazide (tthH) was prepared by the same procedure as adopted in the case of furan-2-thiohydrazide by published method16. Thiophen-2-thiohydrazones were prepared by refluxing 0.025mol of appropriate aldehyde or ketone with methanolic solution (60ml) of thiophene-2-thiohydrazide (0.025mol; 3.95gr). On cooling, the thiohydrazones, separated were filtered washed with cold methanol and recystallised from hot methanol or hot benzene. The reactions in the process are given below:-
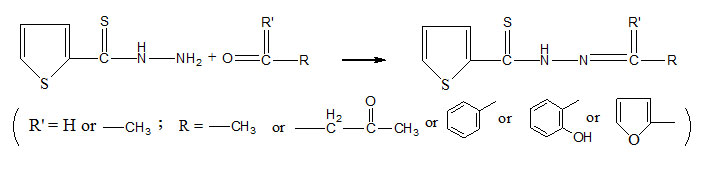
Table 1: Analytical data of prepared ligands are summerised
|
Ligands |
Colour |
M.P (°C) |
Analysis % Found (Calc) |
|||
|
C |
H |
N |
S |
|||
|
Thiophene-2-thiohydrazide (Htth) |
Flaky white |
146 |
37.35 (37.97) |
3.68 (3.80) |
17.59 (17.72) |
40.46 (40.51) |
|
Acetone-thiophene-2-thiohydrazone(Hactth) |
White |
156 |
48.17 (48.48) |
4.83 (5.05) |
13.96 (14.14) |
32.13 (32.32) |
|
Acetylacetone-thiophene-2-thiohydrazone (Hacactth) |
White |
165 |
49.84 (50.0) |
4.79 (5.0) |
11.41 (11.66) |
26.52 (26.66) |
|
Benzaldehyde-thiophene-2-thiohydrazone(Hbentth) |
Cream |
169 |
58.31 (58.53) |
3.87 (4.06) |
11.17 (11.38) |
25.88 (26.01) |
|
Salicylaldehyde- thiophene-2-thiohydrazone (H2Saltth) |
Cream |
176 |
54.77 (54.96) |
3.67 (3.81) |
10.48 (10.68) |
24.28 (24.42) |
|
2-Hydroxy acetophenone- thiophene-2-thiohydrazone (H2aptth) |
Light cream |
179 |
56.31 (56.52) |
4.17 (4.34) |
9.02 (10.14) |
23.03 (23.18) |
|
Furfural-thiophene-2-thiohydrazone (Hftth) |
Cream |
171 |
50.62 (50.84) |
3.21 (3.38) |
11.67 (11.86) |
26.96 (27.11) |
Preparation of Complexes
[ML2], (M=Ni2+ or Zn2+; LH=Hactth; Hacactth, Hbentth, Hftth) and
[M(LH)2], (LH2=H2Saltth, H2aptth)
An aqueous solution (40-50ml) of the metal chloride (0.005mol) was added slowly with stirring to a hot ethanolic solution (40-50ml) of appropriate ligand (0.005mol), and refluxed for 0.5 to 1 hour, when metal complexes separated gradually. In a few Zn(II), the complexes separated on adding equal volume of water. On, cooling, the products were collected on filter paper, washed with aqueous ethanol and dried over Cacl2 and finally in air over at 60-70oC.
[ML(H2O)].nH2O (M=Ni2+ or Zn2+; LH2=H2Saltth, H2aptth; n=2 or O)
An aqueous solution (40-50ml) of the appropriate metal acetate (0.0015mol) was treated with a hot ethanolic solution of ligand (.0015mol) and refluxed for 1-2 hours and PH was maintained at ≈ 7-8 by adding aqueous solution of sodium acetate. The solid products were collected on a filter paper, washed with aqueous ethanol, dried over CaCl2 and finally in an air oven (60-70°C).
[ML(NH3)], (M=Ni2+ or Zn2+; LH2=H2Saltth; H2aptth)
By carrying out similar reactions as above in the presence of 15% NH4OH solution (PH≈9), yielded complexes of composition [ML(NH3)], which was collected on a filter paper, washed with ethanol and dried as above.
[ML.Py] ([ML2], (M=Ni2+ or Zn2+; LH2=H2Saltth; H2aptth)
An aqeous Solution (50ml) of metal chloride (0.0015mol) was treated with pyridine (3-4ml) and the resulting solution was treated with hot ethanolic solution (50ml) of appropriate ligand (0.0015mol) and refluxed for 1-2 hours, when mixed ligand complexes separated out. The solid products were washed with aqueous ethanol and dried over KOH+CaCL2.
The analytical results and physical data of complexes are recorded in Table-2, the UV-vis results are given in Table-3.
Results and Discussion
The analytical data recorded in Table-2, are fully consistent with the formulation of complexes. Thermogravimetric analysis show that, the complexes are stable below 180-200°C, some complexes melt after 200°C but majority of them start decomposing slowly above 210-220°C. the complexes are non-hygroscopic and insoluble in water, Sparingly soluble in methanol, ethanol but highly soluble in DMF, DMSO and dioxane. The negligible molar conductivities in DMF (5-10 ohm-1cm2mol-1) suggest that the complexes are non electrolytes. Except [Ni(Saltth).(H2O)] and [Ni(aptth)H2O], which are feebly paramagnetic , the other complexes are diamagnetic. A minor paramagnetism of these two complexes can be attributed to partial population of a spin-triplet state close to idealised ground state 1A1g for D4h symmetry.18 The diamagnetism of all other Ni(II) complexes indicate their square planar geometry.18
The preference for tetrahedral stereochemistry for complexes of d10 electronic system and observed metal ligand stereochemistry19 indicate tetrahedral structure for the Zn(II) complexes which were found diamagnetic.
Table 2: Analytical and physical data of complexes
|
Complexes |
Colour |
Analysis%, found (Cal) |
µeff (B.M.) |
ΛM (ohm-1 cm2 mol-1) |
|||
|
M |
C |
N |
S |
||||
|
[Ni(actth)2] |
Brown |
12.78 (12.88) |
42.37 (42.66) |
12.26 (12.44) |
28.22 (28.44) |
Dia |
5 |
|
[Zn(actth)2] |
Light yellow |
14.04 (14.22) |
41.72 (42.01) |
11.96 (12.25) |
28.17 (2800) |
Dia |
6 |
|
[Ni(acactth)2] |
Reddish brown |
10.62 (10.86) |
44.71 (44.94) |
10.27 (10.48) |
23.19 (23.40) |
Dia |
6 |
|
[Zn(acactth)2] |
Yellow |
11.82 (12.01) |
44.16 (44.36) |
10.18 (10.35) |
23.42 (23.65) |
Dia |
7 |
|
[Ni(bentth)2] |
Orange red |
10.35 (10.62) |
43.76 (43.95) |
12.04 (12.28) |
23.25 (23.44) |
Dia |
5 |
|
[Zn(bentth)2] |
Orange yellow |
11.63 (11.75) |
43.23 (43.39) |
9.89 (10.12) |
23.25 (23.14) |
Dia |
5 |
|
[Ni(HSaltth)2] |
Reddish brown |
9.84 (10.03) |
41.31 (41.52) |
9.49 (9.68) |
21.97 (22.14) |
Dia |
5 |
|
[Zn(HSaltth)2] |
Yellow |
10.93 (11.11) |
40.87 (41.02) |
9.38 (9.57) |
21.72 (21.88) |
Dia |
7 |
|
[Ni(Haptth)2] |
Brick red |
9.33 (9.57) |
51.24 (51.48) |
9.07 (9.24) |
20.95 (21.12) |
Dia |
6 |
|
[Zn(Haptth)2] |
Yellow |
10.41 (10.60) |
50.65 (50.89) |
9.05 (9.13) |
20.68 (20.88) |
Dia |
7 |
|
[Ni(ftth)2] |
Brown |
10.74 (11.02) |
45.38 (45.62) |
10.51 (10.64) |
24.13 (24.33) |
Dia |
5 |
|
[Zn(ftth)2] |
Yellow |
11.91 (12.19) |
44.82 (45.02) |
10.35 (10.50) |
23.87 (24.01) |
Dia |
6 |
|
[Ni(Saltth)(H2O)] |
Brown |
16.97 (17.26) |
42.62 (42.85) |
8.19 (8.33) |
18.82 (19.04) |
0.62 |
5 |
|
[Ni(aptth)(H2O)].2H2O |
Brick red |
14.87 (15.02) |
40.22 (40.41) |
7.03 (7.25) |
16.24 (16.58) |
0.65 |
6 |
|
[Zn(Saltth)(H2O)] |
cream |
18.71 (18.95) |
41.74 (41.98) |
7.02 (8.16) |
18.49 (18.65) |
Dia |
7 |
|
[Zn(aptth)2)(H2O)].2H2O |
Light Yellow |
16.21 (16.53) |
39.33 (39.69) |
6.95 (7.12) |
16.07 (16.28) |
Dia |
5 |
|
[Ni(Saltth)(NH3)] |
Red |
17.14 (17.31) |
42.81 (42.98) |
12.32 (12.53) |
18.87 (19.10) |
Dia |
5 |
|
[Ni(aptth) (NH3)] |
Reddish brown |
16.45 (16.61) |
44.38 (44.69) |
11.83 (12.03) |
18.08 (18.33) |
Dia |
5 |
|
[Zn(Saltth) (NH3)] |
yellow |
18.77 (19.00) |
41.82 (42.10) |
11.98 (12.28) |
18.53 (18.71) |
Dia |
5 |
|
[Zn(aptth) (NH3)] |
Yellow |
18.05 (18.25) |
43.64 (43.82) |
11.57 (11.79) |
17.83 (17.97) |
Dia |
5 |
|
[Ni(Saltth)Py] |
Red |
14.47 (14.60) |
36.03 (36.27) |
10.34 (10.57) |
15.91 (16.12) |
Dia |
5 |
|
[Ni(aptth)Py] |
Reddish brown |
13.93 (14.11) |
37.76 (37.95) |
9.94 (10.21) |
15.29 (15.57) |
Dia |
6 |
|
[Zn(Saltth)Py] |
Light Yellow |
15.88 (16.08) |
35.45 (35.64) |
10.24 (10.39) |
15.67 (15.84) |
Dia |
5 |
|
[Zn(aptth)Py] |
yellow |
15.32 (15.55) |
37.06 (37.32) |
9.88 (10.04) |
15.17 (15.31) |
Dia |
6 |
Table 3: Electronic reflectance band position in nm
|
Complexes |
Electronic band positions in nm. |
Assignments |
|
[Ni(actth)2] |
360sbr, 375br, 440sh |
π – π*, C –T, 1A1g → 1B1g |
|
[Ni(acactth)2] |
375sbr, 445m, 560m |
π – π*, 1A1g → 1B1g , 1A1g → 1A2g |
|
[Ni(bentth)2] |
380br, 450m, 495m |
π – π*, 1A1g → 1B1g , 1A1g → 1A2g |
|
[Ni(HSaltth)2] |
390br, 450m |
π – π*, 1A1g → 1B1g |
|
[Ni(Haptth)2] |
250s, 305br, 365br, 460sh |
π – π*, n – π*, C –T, 1A1g → 1B1g |
|
[Ni(ftth)2] |
262s, 317br, 450sh |
π – π*, n – π*, 1A1g → 1B1g |
|
[Ni(Saltth)(H2O)] |
270s, 380sbr, 465sh, 580br |
π – π*, n – π*, 1A1g → 1B1g , 1A1g → 1A2g |
|
[Ni(Saltth)(NH3)] |
260s, 325br, 450sh |
π – π*, C –T, 1A1g → 1B1g |
|
[Ni(aptth) (NH3)] |
262s, 321br, 455sh |
π – π*, C –T, 1A1g → 1B1g |
|
[Ni(Saltth)Py] |
235s, 305s, 465sh,500m |
π – π*, C –T, 1A1g → 1B1g , 1A1g → 1A2g |
|
[NI(aptth)Py] |
240s, 310s, 472sh, 505m |
π – π*, C –T, 1A1g → 1B1g , 1A1g → 1A2g |
Electronic Spectral Studies
The solid reflectance spectra of Ni(II) complexes below 400nm display strong absorption attributable to charge transfer band or ligand absorptions. A weak band or shoulder in the regions 420-480nm and 500-600nm are assignable to 1A1g → 1B1g and 1A1g → 1A2g transitions respectively in a planar arrangement of ligand molecules around Ni(II) atom in D4H symmetry.8, 20-21 The thioanide bands of ligands observed in the regions ≈475nm suffers blue Shift in the complexes due to thioenolisation21-24 and broadens considerably probably due to the overlap of ligand to metal charge transfer transitions.
IR Spectral Studies
The absence of any band in the region 2500-2600cm-1 due to ν(SH) shows that all the ligands exist in the thioketo form, which is also supported by 1HNMR studies. The IR absorption bands of νNH in the region 3200-3400cm-1 of the free ligands dissapear in complexes, which indicates that the ligands are deprotonated in complexes. The thioamide band IV of the free ligands (865-935cm-1) which has major contribution from νC=S vibration is observed at 705±20 cm-1 in their complexes, indicating the coordination through the deprotonated thiol sulphur atom.7-8
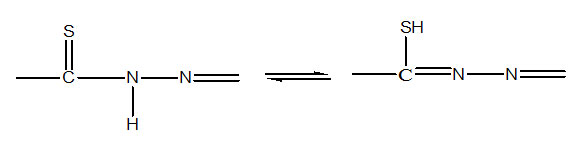
Along with this the νC=N band of agomethine group of ligand (1625±5 cm-1) is observed at lower frequency by 15-10 cm-1 as strong band in the complexes, which indicates coordination of azomethine group. This is further substantiated by the appearance of azine chromophores 22 (>C=N—N=C<) around 1605-1600 cm-1 in the complexes. A broad band at 2950-3150 cm-1 for bis ligated complexes of thiophene-2-thiohydrazones of salicyladehyde and 2-hydroxyacetophenone having composition [M(LH)2] (M=Ni2+, or Zn2+, LH2=H2Saltth; H2aptth) is absent in their monoligated complexes of compositions [ML(H2O]; [ML(NH3)] and [MLPy], which indicates that phenolic OH of salicyladehyde (or 2-hydroxy acetophenone) part is retained in bis-chelates and involved in hydrogen bonding while deprotonotated and coordinated to metal in monoligated complexes. The hydrated complexes [ML(H2O)] (M=Ni2+, or Zn2+, LH2=H2Saltth; H2aptth) display a broad band near 3350 cm-1 attributed to water molecules. The rocking band of H2O is not observed for coordinated water which indicates lattice nature of H2O in the complexes.23 Drastic condition required to dehydrate these complexes also favor a structure containing coordinated water molecule in these complexes.
The coordination of NH3 in the complexes of composition [ML(NH3)] (M=Ni2+, or Zn2+, LH2=H2Saltth; H2aptth) is indicated by the appearance of bands as 3300-3100, 1590, 1280, & 850 cm-1 assignable to νNH3 , δ NH3 and ρ NH3 respectively. In the pyridine complexes of composition [ML(Py)] (M=Ni2+, or Zn2+, LH2=H2Saltth; H2aptth) the pyridine ring νC=N band is observed at 1595±cm-1 in the complexes. The pyridine ring breathing mode of vibrations observed at 1020±5 cm-1 are attributed to coordinated nature of pyridine molecule.24
The bis ligated complexes of acetone and acetylacetone of composition [ML2] (M=Ni2+, or Zn2+, LH=Hactth; Hacactth), display vibrations between 1680-1720cm-1 indicating that carbonyl oxygen is completely free and uncoordinated in complexes. In far infrared region all the complexes display a number of bands at 460-410, 400-380, 360-320cm-1 which are tentatively assigned to M-O, M-N and M-S stretching vibrations21, 25-26 respectively.
So it is found that in slightly alkaline medium (PH≈9) the ligands, salicylaldehyde thiophene-2-thiohydrszone (H2saltth) and 2-hydroxyacetophenone thiophene-2-thiohydrazone (H2aptth) react with Ni(II) or Zn(II) salt giving rise to monoligated complexes of composition [ML(H2O)], [ML(NH3) & [ML(PY)], thus behaving in a dibasic tridentate fashion and bonding takes place through O, N and S – atoms. In neutral or feebly alkaline conditions these ligands and other ligands also found to act in a bidentate monobasic way bonding through azomethine N-atom and sulphur atom (via thioenolisation) keeping the phenolic –OH free.
1HNMR Studies
The signals due to –NH proton δ(≈11.8)ppm and phenolic –OH proton δ(≈12.4)ppm of ligands (H2Saltth) and( H2aptth) disappear in its monoligated complexes of composition [ML(H2O)], [ML(NH3) & [ML(Py)], which shows the dibasic tridentate nature of these ligands in complexes, bonding through O, N & S atoms.27-28 In the neutral bis chelates signals due to –NH proton δ(11-12)ppm of ligands disappear in complexes, which suggests thioenolisation & coordination through deprotonateed thiol sulphur atom. In neutral bis chelates of (H2Saltth) & (H2aptth), the signals due to phenolic -OH appearing in the range δ(≈12.4)ppm remains unaffected in complexes and its downfield shift than normal phenolic -OH, shows H-bonding. The chemical shift of thiophene ring protons δ(7.1-7.3)ppm of ligands undergo slight downfield shift δ(7.2-7.5) on complexation.
Biological Studies
Monoligated pyridine complex of salicyaldehyde thiophene-2-thiohydrazone [M(Saltth)(Py)] and bis ligated complexes of furfural thiophene-2-thiohyrazone [M(ftth)2]; (M=Ni2+, or Zn2+) have also been tested for antifungal activity. The screening of all the complexes were carried out against two fungal species viz. Aspergillus niger and Rhizopus at three concentrations. Viz 25, 50 and 100µg ml-1.All the complexes are found to be active and show a higher percentage of inhibition at lower concentrations. The complexes are found biologically more active than ligands.
Acknowledgements
The authors are thankful to Professor P. C. S. Verma and Gaurav Pandey, department of Microbiology, L. S. College, B. R. A. B. U. Muzaffarpur for providing necessary facilities to find the biological activities of complexes.
Conflict of Interest
There is no conflict of interest.
References
- Lobana S, Kumari P, Sharma R, Castineiras A, Butcher R. J., Akitsu T and Aritake Y, Dalton Trans, 2011, 40, 3219-3228,
CrossRef - Lobana S, Sharma R, Bawa G and Khanna S, Coord. Chem. Rev, 1993, 123, 49-71,
- Casasm S, Garsia-Tasende M. S and Sordo J, Coord. Chem. Rev, 2000, 209, 197-261.
CrossRef - Kovale-Demertzi D, Yadav P.N, Demertzis M.A, Jasioki J.P, Andreadaki F.J and Kostas I.D, Teahedron Lett, 2004, 45, 2923-2926.
CrossRef - Singh R.B and Ishii I, Cryst Rev; Chem 1991.22, 381-401.
CrossRef - Biswas P.K, Chaudhuri N.R., Chem. Soc,Dalton Trans 1981, 2385.
CrossRef - Kumar A, Jha A.K, Majumdar N, Yadav S.N and Mishra L.K, Indian Chem. Soc.; 1997, Vol-74, 485-486.
- Keshri B. N and Mishra L. K. , J. Indian. Chem. Soc. 1981, Sect-A, 20,
- Mishra L. K , J. Indian. Chem. Soc.; 1981, 58, 1198.
- Dwivedi A. K. , Ojha V. S. , Tiwari H. N. and Mishra . L. K , J. Indian. Chem. Soc. ; 1995, 72, 403-405.
- Hasain S. S. and Piggot B. Biochem, Biophys, Res. Commun., 1983, 112, 279.
- Wilkinson Ed. Sir G. , ‘Comprehensive coordination chemistry’ , Pergamon Press, 1987, Vol-6, 112.
- Thauer R. K, Diekert G. and Schonheit P. , Trends Biochem, , 1980, 304.
CrossRef - Kaim W. and Schwederski B. , Bio-inorganic Chemistry, 1994, 242-266.
CrossRef - Vogel Arthur. I. ‘A text book of quantitative inorganic analysis’, 1962, 389-390, 480.
- Shome S. C. , Nandy S. , Guhathakurta A. , Ghose N. C. , Das H. R. and Ganagopadhayay P. K. Microchimica Acta, 1978, 70, 343.
CrossRef - Dave L. D. and Thumpy S. K. , Indian J Chem, 1981. Sec A, 20,
- Figgis B. N. and Lewels J. , Inorg. Chem., 1965, 6, 37.
- Cotton F. A. and Wilkinson G. ‘ Advanced inorganic chemistry, A. comprehensive Text, Interscience’ New York, 1966, 610-621.
- Gray H. B and Bathausen C. J. , J Am. Chem. Soc, 1963, 85, 260.
CrossRef - Tarafder M.T.H. and Ali M. , Can. J. Chem. , 1978, 56, 2000.
CrossRef - Biradar N. S and Kulkarni V.H. , Inorg. nucl. Chem. 1971, 33, 2451.
CrossRef - Arbindakshan K. K. , Indian J. Chem. , Sec-A, 1987, 26, 241.
- Lane T. J. Nakagawa I. , Walter J. L. and Kandathil A. J. , Nucl. Chem., 1961, 18,79.
- Speca A. N. , Karayannis N. M. and Pytlewski L. L. , Chim Acta, 1976, 17, 19.
CrossRef - Ali M. A. , Livigstone S. E. and Philips D. J. Chim. Acta, 1971, 5, 119.
- Shetty U. N , Revankar V. K. , Mahale V. B. , Indian Acad. Sci. ( Chem. Sci.) 1997, 7, 109.
- Prichard G. , John R. , org. chem. , 1970 , 35.

This work is licensed under a Creative Commons Attribution 4.0 International License.










