Sunlight Mediated Synthesis of Silver Nanoparticles by Bacillus SP and Its Antibacterial Property
Chemistry and Environmental Science Department, College of Arts and Sciences, Nueva Ecija University of Science and Technology, Cabanatuan City, Nueva Ecija, 3100, Philippines
Corresponding Author E-mail: kgmartin@up.edu.ph
DOI : http://dx.doi.org/10.13005/ojc/360309
Article Received on : 16 May 2020
Article Accepted on : 17 Jun 2020
Article Published : 30 Jun 2020
Development of antibiotic resistant strains in pathogenic bacteria is an alarming issue worldwide. Utilization of silver nanoparticles (AgNPs) could be possible way to address this concern but the conventional methods of producing AgNPs are toxic to the environment and expensive. This study demonstrates a cleaner and cheaper way of synthesizing AgNPs using silver tolerant bacterium and sunlight. Isolate I-NEUST was isolated from a local junkshop at Cabanatuan City, Nueva Ecija, Philippines was able to tolerate 3.5 mM of AgNO3 and was found to be closely related to bacilli species.AgNPs were successfully synthesized with a size of 63.8 nm and concentration of 4.44 mM. Silver nanoparticles exhibited considerable antibacterial activity against multidrug human pathogens. The effect was more distinct on Gram-negative bacterium Escherichia coli ATCC 25922. Nanoparticles also showed prominent activity on Gram-positive bacterium Staphylococcus aureus ATCC 25923. Hence, the present study demonstrates that biologically synthesized nanoparticle could be employed for developing antibacterial drug.
KEYWORDS:Antibacterial Property; Bacillus Megaterium; Silver Nanoparticles; Silver Tolerant; Sunlight Mediated
Download this article as:| Copy the following to cite this article: Martin K. D. G, Padilla K. G. V. Sunlight Mediated Synthesis of Silver Nanoparticles by Bacillus SP and Its Antibacterial Property. Orient J Chem 2020;36(3). |
| Copy the following to cite this URL: Martin K. D. G, Padilla K. G. V. Sunlight Mediated Synthesis of Silver Nanoparticles by Bacillus SP and Its Antibacterial Property. Orient J Chem 2020;36(3). Available from: https://bit.ly/31xkJTh |
Introduction
Antibiotics are becoming less efficient in combating microbial infection due to the rapid development of antibiotic resistant strains. One possible solution is the utilization of nanoparticles (NP) which have caught the attention of many researchers in battling pathogenic strains of bacteria without the fear for developing antibiotic resistant strains.1 Nowadays, nanoparticles, specifically silver NPs are used for possible medical applications like antibacterial, antifungal, anti-inflammatory, wound healing, antiviral, antioxidant, antitumor, and even treatment for cancer 2. In addition, silver NPs are applied in medical devices, molecular diagnostics, photonic devices, sensors, textile, and water purifier.3 Different products like wound dressings, medical devices, dental materials, water disinfectant, and room sprays can be integrated with nanoparticles to prevent bacterial infection.4 With the promising benefits and uses of nanoparticles, one problem arises. Most of the methods involved in the synthesis of nanomaterials are inefficient and cost demanding. Chemical synthesis of nanoparticles produces toxic substances that can be incorporated on the surface of NPs which has an opposing effect when used for medical applications.5 Physical methods which uses evaporation-condensation to produce NPs, although accurate in producing stable and specific size of nanoparticles is very expensive.1 This creates a demand in developing sustainable, less toxic, and safe techniques in the synthesis of nanoparticles. An alternative way is using biological samples like microorganisms, which is nontoxic, clean, and ecofriendly way of achieving the goal of synthesizing nanoparticles.6 In addition to the utilization of microorganisms, photo-irradiation is being implemented to assist the synthesis process, which is cost-effective, non-toxic, and renewable.7
Utilization of silver-tolerant bacteria coupled with photo-irradiation is one of the cleaner and efficient ways for nanoparticle production. Silver-tolerant bacteria can be found in environment with high concentration of heavy metals. Presence of heavy metals reduces the population of bacteria, but long exposure creates metal tolerant strains.8 The tolerance, high affinity, and the ability to reduce metal ions into metal nanoparticles allowed the production of diverse morphology of nanoparticles by microorganisms.9 Reported silver-tolerant bacterium, Pseudomonas stutzeri, was found to accumulate silver nanoparticles, proving the capability of microorganisms to produce AgNPs10. In the study of Adriano et al (2018), silver-tolerant Bacillus cereus, Bacillus subtilis, Bacillus flexus, Bacillus thuringiensis, Alcaligenes faecalis, Achromobacter sp. and Ochrobactrum sp. were isolated from a major Philippine landfill which were used to synthesize AgNP.11 In addition, another silver-tolerant Bacillus sp. was isolated from the atmosphere and successfully synthesized AgNPs.10
Most of the reported silver-tolerant bacteria are members of bacillus species. Bacillus megaterium, a gram-positive and aerobic spore forming bacterium, which can be found in diverse environment ranging from soil to seawater was also used to synthesize AgNPs. In the study conducted by Karkaj et al (2013)AgNPs with sizes ranging from 10-60 nm were synthesized using Bacillus megaterium.12 In addition, a study of Saravanan et al (2011)also utilized Bacillus megaterium in producing AgNPs.13
In this study, silver-tolerant bacterium was isolated from a local junkshop in Cabanatuan City, Nueva Ecija, Philippines and identified using molecular techniques. Isolated bacterium was grown for silver NP synthesis which was catalyzed through exposure to sunlight. In addition, antibacterial property of synthesized silver nanoparticles was tested against gram-negative and gram-positive bacteria.
Materials and Methods
Sample Collection
Methods for soil sampling was adopted from the methods of Piotrowska-Seget et al (2005).14 Soil samples were from identified junkshop in Cabanatuan City, Nueva Ecija, Philippines and was stored in polyethylene bags. Samples were collected from the top layer (0-10 cm) where metals are directly dumped.
Isolation of Silver Tolerant Bacteria
Soil samples were homogenized and diluted using distilled water. Ten grams of sample weighed and dissolved in 90 ml of dH2O, followed by serial dilution until desired concentration (10-6 dilution) was achieved.15 Isolation of tolerant bacteria used 0.5 up to 3.5 mM AgNO3 and nutrient agar. Solution of AgNO3 was used to prepare the nutrient agar were the bacterium was grown.14 Diluted soil samples (0.5 ml) were applied to prepared agar plates in triplicates. Plates were stored in dark with a temperature of 25°C. After one week, colonies were isolated and purified using standard microbial purification techniques.16
Identification of Silver Tolerant Bacterium
Isolated bacterium was identified using 16s rRNA. Colonies of the bacterium was sent to Macrogen Inc., South Korea where the DNA was extracted from bacterial samples and amplified using universal 16s primers. Amplified DNA samples was then sequenced using Sanger sequencing method. Basic Local Alignment Search Tool or BLAST was used to check the identity of bacterial isolates. A phylogenetic tree was then constructed using Molecular Evolutionary Genetics Analysis (MEGA) X software.17
Synthesis and Characterization of Silver Nanoparticles
Isolated silver tolerant bacterium was used to synthesize silver nanoparticles from 3.5 mM of AgNO3. Isolated bacteria were grown in a 50 ml of nutrient broth and was kept in the dark for 7 days. Precipitate of the bacterium was harvested through centrifugation of the broth at 10 000 rpm for 10 minutes, the collected precipitate was then washed with distilled water, this step was repeated for five times to avoid contamination from nutrient broth during the synthesis of nanoparticles. Silver nitrate was added to the bacterial precipitate and stored in darkness at room temperature for 24 hours. After the incubation period, the solution of AgNO3 and bacteria was exposed to sunlight (12 PM) for 10 minutes, color change from clear to a yellow-brown solution indicates successful synthesis of silver nanoparticles.18 And to determine the size and concentration of the nanoparticles, absorption of the solution from 300 nm to 700 nm was determined using a UV-Vis Spectrophotometer.19
Antibacterial Properties of Silver Nanoparticles
Antibacterial activity of synthesized silver nanoparticles was screened against Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923 using standard-agar disc diffusion method. The gram-negative bacteria E. coli ATCC 25922 and gram-positive bacteria S. aureus ATCC 25923 were chosen based on their clinical and pharmacological importance. Three (3) to five (5) colonies of E. coli ATCC 25922 and S. aureus ATCC 25923 were grown for 16 to 18 hours in nutrient broth at 35℃ and was transferred to sterile distilled water and the turbidity was adjusted to McFarland 0.5 standard (~1.5 x 108 CFU/ml). Mueller Hinton Agar (MHA) plates were inoculated using a sterile cotton swab moistened with the standardized culture. Streaking of the entire surface was done three times accompanied by rotation at every application to cover all areas.20
Sterile 6 mm paper discs were placed on a sterile empty Petri plate where about 20 μL of silver nanoparticle solution was pipetted and allowed to stand for a few minutes until excess liquid has flowed out. Using sterile forceps, infused discs was then transferred carefully onto previously inoculated MHA plates equidistant to each other. Antibacterial activity was detected after an incubation of 24–48 h at 37°C. Norfloxacin (5 μg; Hi-Media SD184) for E. coli ATCC 25922 and Erythromycin (15 μg; Hi-Media SD013) for S. aureus ATCC 25923 served as the positive controls. Plates were prepared in triplicates. The presence of a clear or translucent zone of inhibition around the discs were used as an indicator of positive antibacterial property of silver nanoparticles.20
Results
Identification of Silver Tolerant Bacterium
The isolated bacterium was able to withstand silver ions from 0.5 mM of AgNO3 up to 3.5 mM. With the use of DNA barcoding technique, the bacterium was identified to be closely related to Bacillus megaterium. MEGA X was used to create a phylogenetic tree utilizing the 16s rRNA sequence from Isolate I-NEUST. Isolate I-NEUST, as shown in the figure below, paired with the Bacillus megaterium.
 |
Figure 1: Constructed Phylogenetic tree of Isolate I-NEUST using MEGA X. |
Synthesis and Characterization of Silver Nanoparticles
Precipitates from the Isolate I-NEUST was used to produce AgNPs. The solution that contains the bacterial precipitates and silver nitrate (b), when exposed to sunlight for ten minutes, change its color from clear to brown solution while the solution of silver nitrate (a) remained clear as shown in figure 2. Change in the color of silver nitrate solutions indicate the presence of silver nanoparticles.
 |
Figure 2: Color change in the AgNO3 containing solutions after exposing to 10-minute sunlight, |
Absorption of the solution from wavelengths 300 nm – 700 nm was determined using UV-Vis spectrophotometer to confirm the presence of AgNPs. As shown in the graph below, the solution has a lambda max at 436 nm, peaks at around 400-450 nm indicate the presence of AgNPs. This confirms the successful synthesis of nanoparticles using silver-tolerant Isolate I-NEUST with sunlight exposure. Size and concentration of silver nanoparticles were determined using the absorbance at 436 nm. The size of produced silver nanoparticles was 63.8 nm with a concentration of 4.44 mM as shown in table 1.
Table 1: Size and concentration of AgNPs synthesized using Isolate I-NEUST
|
λ(max) |
Absorption |
Size |
Concentration |
|
|
AgNP |
436 |
0.90564 |
63.8 nm |
4.44 mM |
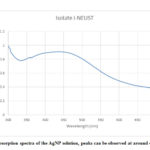 |
Figure 3: Absorption spectra of the AgNP solution, peaks can be observed at around 400 to 450 nm |
Antibacterial Properties of Silver Nanoparticles
Antibacterial property of the silver nanoparticles was tested against two multidrug-resistant human pathogens such as E. coli ATCC 25922 and S. aureus ATCC 25923. After the incubation period, zone of inhibition was observed in the disks containing solutions of nanoparticles on both plates streaked with E. coli ATCC 25922 (a) and S. aureus ATCC 25923 (b) as shown in figure 4. This confirms the presence of antibacterial property in the bacterial synthesized silver nanoparticle.
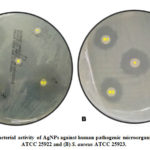 |
Figure 4: Antibacterial activity of AgNPs against human pathogenic microorganisms |
Discussion
Isolate I-NEUST was found to be closely related to bacillus species especially Bacillus megaterium. Studies related to isolation of silver tolerant bacillus species are very limited, but some researches have shown that members of the mentioned bacterial family are capable of synthesizing silver nanoparticles. Bacillus megaterium, in the study of Saravanan et al (2011),13 successfully synthesized silver nanoparticles with sizes range of 80-98.56 nm which is relatively larger compared from the silver nanoparticles produced by Isolate I-NEUST which is 63.8 nm.
Presence of AgNPs in the solution was detected using UV Vis spectrophotometer. AgNPs are thought to be formed through the accumulation of Ag ions on the cell wall of the bacterium through biosorption followed by the reduction of Ag ions which forms the nanoparticle. The diverse biomolecules such as proteins and polysaccharide produced by Bacillus megaterium perform important role in stabilizing AgNPs. The cell wall of the bacterium act as the capping agent for AgNPs which prevents them from aggregation and adds stability to the nanoparticle .21
The bacterial solution was also subjected to sunlight for ten minutes. There are few researches which utilizes both sunlight and bacterial samples in the synthesis of AgNPs. This study reports the successful sunlight mediated synthesis of AgNPs using a silver tolerant bacterium Isolate I-NEUST. In the study of Manikprabhu et al (2016), sunlight mediated synthesis was also used with the utilization of a novel actinobacterium, Sinomonas mesophila MPKL 26 producing 4-50 nm AgNPs.18 A similar research by Saravanan et al (2011) also used Bacillus megaterium to synthesize 80–98.56 nm AgNPs although the bacterium species used was not reported to be silver tolerant and the method used was not sunlight mediated.13 Presence of sunlight act as a catalyst for the reduction of silver nitrate to silver nanoparticles, wherein metabolites like of Isolate I-NEUST could be probable source of reducing/capping agent during the synthesis of nanoparticles.18
Antibacterial property of the silver nanoparticles was shown against E. coli ATCC 25922 and S. aureus ATCC 25923. According to Geethalakshmi and Sarada (2012), the prominent inhibitory effect of silver has been known for a long time and used for various medical applications. In order to examine the antibacterial activity of silver nanoparticles, the disc diffusion method was employed (Figure 4).22 In the case of E. coli ATCC 25922 plate (Figure 4.a), the three replicates filled with AgNPs showed maximum inhibitory zone comparable to the Norfloxacin as a positive control which indicates that silver nanoparticles exhibited antibacterial activity against the bacterium. As for the case of S. aureus ATCC 25923 plate (Figure 4.b), it showed minimum inhibitory zone compared to E. coli ATCC 2592 but as good as Erythromycin, the positive control used for this bacterium. Still, Silver NPs showing inhibitory area to both pathogenic bacteria encompassed the antibacterial activity of silver nanoparticles synthesized from isolated silver tolerant bacterium. Accordingly, it has been reported that antimicrobial activity is dose and size dependent and more obvious against Gram negative than Gram positive bacteria. 23 Presumably, Prasad et al. (2015) reported that the Gram negative cell wall, composed of thin peptidoglycan layer, is more susceptible to AgNPs permeation when compared to the Gram positive cell wall, which is consisted of a thicker peptidoglycan layer, creating an effective barrier against the AgNPs penetration, assumption that is in agreement with previous reports.24 Marambio-Jones and Hoek (2010) suggested that Ag+ ions of AgNPs attached to the negatively charged cell surface alter the physical and chemical properties of cell membranes and disturb important functions such as permeability, osmoregulation, electron transport, and respiration.25
Aside from these, there are various mechanisms proposed in the literature for the antimicrobial effect of SNPs. Patil et al. (2012) claimed that the cell death arising out of exposure to SNPs might be due to the cytoplasmic membrane disorganization and the consequent leakage of various biomolecules such as amino acids, protein and carbohydrates.26 Raja et al. (2017) concluded that the high conductivity of cells treated with SNPs was due to the breakage of double-stranded DNA molecules present in the bacteria. Moreover, Van Viet et al., (2018) cited that the excellent antibacterial properties of Ag NPs can be explained by the chemical property of Ag+ ions which strongly bond with peptidoglycan – a component in the bacterial cell wall and inhibit the oxygen transport through the cells. In addition, Ag+ ions combine with bacterial cell wall and replace H+ in the S-H group (cysteine amino acid) of the sulfur containing enzyme to form (–S–Ag) group, leading to damage the cell respiratory.27
Silver NPs induces formation of free radicals which causes cell membrane damage in the bacteria.28 Toxicity of silver NPs in Escherichia coli is due to the leakage of reducing sugar and proteins resulting to the shift of respiratory chain dehydrogenase into inactive state, which accounted to the destruction of membrane’s permeability.29 Formation of “pits” in the cell membrane of the bacteria caused leakage of cell contents leading to cell death.28
This study showed that the sunlight mediated synthesis of AgNPs is possible using isolated species. Synthesized AgNPs showed antibacterial properties which can be used in the medical field for battling antibiotic resistant strains. This study proposes a cleaner and greener way of synthesizing AgNPs which utilizes bacterial species, specifically silver tolerant species, together with sunlight as catalyst.
Acknowledgements
We would like to acknowledge Ma’am Danilet Vi M. Mendoza, Sir Darwin F. Reyes, Dyanne Jane Cid Duldulao, and Noel Coloma for their support and advice. Thank you.
Confict of Interest
The authors declare that there is no conflict of interest in this work with regards to publication.
References
- Galvez, A. M., Ramos, K. M., Teja, A. J., & Baculi, R. Journal of Microbiology, Biotechnology and Food Sciences, 2019, 2019, 970-978.
- Brahmachari, G., Sarkar, S., Ghosh, R., Barman, S., Mandal, N. C., Jash, S. K., … & Roy, R. Organic and medicinal chemistry letters, 2014, 4(1), 18.
- Singh, R., Shedbalkar, U. U., Wadhwani, S. A., & Chopade, B. A. Applied microbiology and biotechnology, 2015, 99(11), 4579-4593.
- Hamouda, I. M. Journal of biomedical research, 2012, 26(3), 143-151.
- Ramya, M., & Subapriya, M. S. Int J Pharm Med Biol Sci, 2012, 1(1), 54-61.
- Iravani, S. International scholarly research notices, 2014, 2014.
- Mathew, S., Prakash, A., & Radhakrishnan, E. K. Inorganic and Nano-Metal Chemistry, 2018, 48(2), 139-145.
- Piotrowska-Seget, Z., Cycoń, M., & Kozdroj, J. Applied Soil Ecology, 2005, 28(3), 237-246.
- Javaid, A., Oloketuyi, S. F., Khan, M. M., & Khan, F. BioNanoScience, 2018, 8(1), 43-59.
- Pugazhenthiran, N., Anandan, S., Kathiravan, G., Prakash, N. K. U., Crawford, S., & Ashokkumar, M. Journal of Nanoparticle Research, 2009, 11(7), 1811.
- Adriano, J. S., Oyong, G. G., Cabrera, E. C., & Janairo, J. I. B. Ecological Chemistry and Engineering S, 2018, 25(3), 469-485.
- Karkaj, O. S., Salouti, M., Zanjani, R. S., & Derakhshan, F. K. Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry, 2013, 43(7), 903-906.-906.
- Saravanan, M., Vemu, A. K., & Barik, S. K. Colloids and Surfaces B: Biointerfaces, 2011, 88(1), 325-331.
- Piotrowska-Seget, Z., Cycoń, M., & Kozdroj, J. Applied Soil Ecology, 2005,28(3), 237-246.
- Margesin, R., & Schinner, F. (Eds.). Manual for soil analysis-monitoring and assessing soil bioremediation(2005) (Vol. 5). Springer Science & Business Media.
- Langaoen, A. F., Manzano, V. J. V., Requilman, E. M. R., Tabardillo, J. M., Maningas, M. B. B., & Calugay, R. J. Aquaculture, Aquarium, Conservation & Legislation, 2005, 11(2), 505-515.
- Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. Molecular biology and evolution, 2018, 35(6), 1547-1549.
- Manikprabhu, D., Cheng, J., Chen, W., Sunkara, A. K., Mane, S. B., Kumar, R., … & Li, W. J. Journal of Photochemistry and Photobiology B: Biology, 2016, 158, 202-205.
- Paramelle, D., Sadovoy, A., Gorelik, S., Free, P., Hobley, J., & Fernig, D. G. Analyst, 2014, 139(19), 4855-4861.
- Padilla, K. G. V., Jacinto, W. R., & Cruz, K. G. J. International Journal of Biology, Pharmacy, & Allied Science, 2018, 7(8): 1537-1550.
- Pantidos, N., & Horsfall, L. E. Journal of Nanomedicine & Nanotechnology, 2014, 5(5), 1.
- Geethalakshmi, R., & Sarada, D. V. L. International journal of nanomedicine, 2012, 7, 5375.
- Singh, M., Singh, S., Prasad, S., & Gambhir, I. S. Digest Journal of Nanomaterials and Biostructures, 2008, 3(3), 115-122.
- Prasad, R., Pandey, R., & Barman, I. Biotechnol Adv, 2015, 27, 76-83.
- Marambio-Jones, C., & Hoek, E. M. Journal of Nanoparticle Research, 2010, 12(5), 1531-1551.
- Patil, S. V., Borase, H. P., Patil, C. D., & Salunke, B. K. Applied biochemistry and biotechnology, 2012, 167(4), 776-790.
- Van Viet, P., Sang, T. T., Bich, N. H. N., & Thi, C. M. Journal of Photochemistry and Photobiology B: Biology, 2018, 182, 108-114.
- Kim, S. H., Lee, H. S., Ryu, D. S., Choi, S. J., & Lee, D. S. Korean J. Microbiol. Biotechnol, 39(1), 2011, 77-85.
- Li, W. R., Xie, X. B., Shi, Q. S., Zeng, H. Y., You-Sheng, O. Y., & Chen, Y. B. Applied microbiology and biotechnology, 2010, 85(4), 1115-1122.

This work is licensed under a Creative Commons Attribution 4.0 International License.










