Phytochemical, Antimicrobial and Total Phenol Test of Coral Plants “Betadin” Leaf Methanol Extract (Jatropha Muitifida Linn)
Hanafi , Septilina Melati Sirait*
, Septilina Melati Sirait* , Sri Redjeki Setyawati
, Sri Redjeki Setyawati and Lilis Sulistiawaty
and Lilis Sulistiawaty
Department of Food Quality Assurance, Polytechnic AKA Bogor, Indonesia
Corresponding Author E-mail: septilinamelati.aka@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/360330
Article Received on : 01 May 2020
Article Accepted on : 14 Jun 2020
Article Published : 16 Jun 2020
Coral Plants leaf was extracted with methanol and carried out by maceration then evaporated with a rotary evaporator. This extract contained several phytochemical compound such as alkaloid, flavonoid, tannin, phenol and terpenoid. Therefore, Coral Plants leaf has an antimicrobial function. Phenol compound has the ability to form complex compound with proteins through hydrogen bonds, which can damage bacterial cell membrane. Total phenol content was quite high in the leaf of 165.20 ± 33 mg GAE / mL. Antibacterial activity test results showed that the methanol extract of Coral Plants leaves can inhibit E. coli gram-negative bacteria with strong inhibition (10-20 mm) and gram-positive bacteria (Bacillus sp) by 12.3 mm. Coral Plants leaves can inhibit the growth of penicillium and Tricodherma fungi, but cannot inhibit the growth of Aspergillus niger fungi. The inhibition against Penicillium was higher (7 mm) compared to Tricodherma of 1.8 - 4.5 mm
KEYWORDS:Antimicrobial; Coral Plants; Leaf; Phytochemical; Total Phenol
Download this article as:| Copy the following to cite this article: Hanafi H, Sirait S. M, Setyawati S. R, Sulistiawaty L. Phytochemical, Antimicrobial and Total Phenol Test of Coral Plants “Betadin” Leaf Methanol Extract (Jatropha Muitifida Linn). Orient J Chem 2020;36(3). |
| Copy the following to cite this URL: Hanafi H, Sirait S. M, Setyawati S. R, Sulistiawaty L. Phytochemical, Antimicrobial and Total Phenol Test of Coral Plants “Betadin” Leaf Methanol Extract (Jatropha Muitifida Linn). Orient J Chem 2020;36(3). Available from: https://bit.ly/2B9WXl5 |
Introduction
The principle “back to nature” is increasingly popular in this modern era, with the advancement of science many people turn to traditional medicine. One of the plants that has benefit as an alternative treatment for disease is “betadin”, Jatropha multifida L. derived from the family Euphorbiaceae which is widely grown in the continent of Africa and Asia. “Betadin” plant (Jatropha multifida L.) is a medicinal plant which is rarely used by people, especially in Indonesia. The benefit of this plant is not widely known by the public, especially in Indonesia. This plant can respond to climatic condition, are able to adapt to various agro-ecological and have accumula ted variations over the years.
This plant has many benefits for life that can combat microbial infections and leishmaniasis. In the future, it has a strong laxative activity. The gum of this plant can provide an inhibiting effect on the flow of blood in the wound incision. The stem extract can be used as a plant-based pesticide controlling pest Plutella xylostella in mustard plants (Brassica juncea L.)1
“Betadin” plants are known contained alkaloid and flavonoid. Flavonoid is secondary metabolite
 |
Figure 1: Coral Plants (Jatropha muitifida Linn.). |
in plants and is polar compound, and therefore soluble in alcohol.2 This secondary metabolite can be used as an antimicrobial, infectious drug in wounds, antifungal, antibacterial, hypo-allergenic, cytotoxic, and anti-hypertensive. Jatropha multifida L. is widely used by people as a wound medicine, this is what causes this plant to be known as betadin plant.5 In addition, flavonoid can be used as antioxidant, which are compound that protect cells against the effect of damage by reactive oxygen.3 Flavonoid can also affect the increase in platelet counts and have anti-cancer, anti-virus, anti-bacterial, anti-inflammatory and anti-allergic bioactivity.5
Liana’s research in 2016 also showed the antibacterial activity of betadin leaf active fraction against Staphylococcus aureus.3 This can be caused by the presence of active ingredient flavonoid and phenol. This result was supported by a study of Nagaharika, et.al in 2013 which showed betadin leaves can be used as anti-inflammatory.4 Phenol compound has the ability to form complex compound with protein through hydrogen bond, which can damage bacterial cell membranes.
From the result of the previous study,3 it was known that the benefit of betadine leaf as a wound drug were related to the antibacterial activity against Staphylococcus aureus and had not been studied much in other types of bacteria or in types of fungi. Chemical compounds, especially phenol compounds, are active antimicrobial substances, so they need to know their levels and are expected to produce different antibacterial and antifungal activities. Therefore, researchers want to explore further about betadin plants whether they have the antibacterial activity of E.coli and Bacillus and types of fungi, such as Aspergilus flavus or Aspergilus niger. In this research, methanol solvent was used to extract betadin leaves, which then determined the total amount of phenol and antibacterial and antifungal activity test through growth inhibitory test.
The aim of this experiment was to obtain information on chemical content, phenol content and antimicrobial activity as an antibacterial and antifungal in extract methanol of betadin leaf (Jatropha muitifida Linn.). The result of this study are expected tp demonstrate the effectiveness of betadin leaf methanol extract in inhibiting microbial growth.
Material and Methods
Materials and Chemicals
Coral Plants “Betadin” (Jatropha mulitifida Linn) leaves, methanol (pa), concentrated Hydrochloric Acid (HCl), ammonia, chloroform, Dragendorf reagent, Mayer reagent, Mg powder, Amyl alcohol, Diethyl ether, Libermen reagent, FeCl3, Agar Nutrient media, Potatos Dextros Agar media, Folin-Ciocalteu reagent, anhydrous Na2CO3, test suspension (bacteria and fungi), penicillin and ketomidazole standards. The tools used were: UV-VIS spectrophotometers, rotary evaporators, ceramic containers, petri dishes, magnetic stirers, vortices, paper discs, centrifuges, incubators, erlenmeyers, goblets, vial bottles, test tubes, ose, bunsen, tweezers, and other glassware.
Coral Plants Leaf Extraction
Coral Plants and stalk leaves were separated and washed, then drained and dried in direct sunlight for 4 hours. A total of 50 grams of simplicia Coral Plants leaf was extracted with a solvent ratio of 1:10 (w / v). The solvent used was methanol. Extraction was carried out as much as 2×24 hours by maceration. The maceration result was filtered, then evaporated with a rotary evaporator at a temperature of 50 oC. Furthermore, the calculation of the yield content of the extract obtained.
Extract yield rate (%) = (Extract weight / Simplification weight) x 100%
Phytochemical Test of Coral Plants Leaf Extract
Alkaloid Identification
A total of 100 mg of Coral Plants leaf methanol extract, added 5 mL of 10% HCl and diluted ammonia to pH 8, then extracted with 20 mL chloroform, then the extract was evaporated. The extract was dissolved with 2 mL HCl 2% and divided into 3 tubes. The first tube was used as a comparison, the second tube was added by Mayer reagent and the third tube was added by Dragendorff reagent. If there is a white precipitate with a Mayer reagent, an orange-red precipitate with a Dragendorf reagent in the sample.7
Flavonoid Identification
As much as 100 mg of methanol extract Coral Plants leaves were dissolved in 100 mL of hot water, then boiled for 5 minutes then filtered. As much as 5 mL of filtrate was added 0.1 mg of Mg powder, 1 mL of concentrated HCL and 1 mL of amyl alcohol and then shaken vigorously. The presence of flavonoids was shown by the formation of red, yellow or orange in the amyl alcohol layer.7
Triterpenoid and Steroid Identification
A total of 100 mg Coral Plants leaf extract was added with 25 mL of diethyl ether and then shaken. The diethyl ether layer was separated and 2-3 Lieberman-Burchard reagents were added. Triterpenoids were present when blue solutions were formed and steroid were present when green solutions are formed7
Saponin Identification
A total of 100 mg Coral Plants leaf extract was added with 10 mL of hot distilled water, cooled, and shaken vigorously for 10 minutes. Saponins were present when a solid foam was formed and on the addition of 1 drop of HCl 2 N (the foam remains stable)7
Tanin Identification
A total of 100 mg Coral Plants leaf extract was extracted using 1 mL ethanol and 1 mL aquadest. The filtrate obtained was then added a few drops of FeCl3 1%. The presence of tannin compounds was indicated by the formation of green, blue or purple7
Antimicrobial Activity Test
Petri dish containing Nutrient agar media was added 200 μL suspension of one of the test bacteria (E.coli, and B.cereus) and then spread using a string. To test the antifungal media used was a PDA (Potatos dextrose Agar) with A.Plavus, and A. niger test fungi. Next, sterile tracing paper (6 mm diameter discs) was prepared to be placed on the surface so that the petri dish was prepared. The discs were previously soaked in the test solution for 30 minutes in a sterile petri dish. Then the incubation process was carried out at 37oC for 24 hours. Observations were made by measuring the zone of resistance formed around paper discs in the form of clear areas using a ruler then recorded. This applied also to standard using antibiotics p.a.
Inhibition Zone = Diameter (clear area + discs) – Diameter of disc paper
Analysis of Total Phenols (Folin Method)
Each sample was piped as much as 2 mL, then macerated with ethanol as much as 3 mL, and shaken. The extract was dropped on a drip plate and added with 5% FeCl3 solution of 3-5 drops. A positive test for the presence of phenolic compounds was shown if the color changes to green, blue, purple or black. Gallic acid stock solution with a concentration of 100 ppm (mg / L), which can be made by dissolving 0.01 g gallic acid in a 100 mL volumetric flask and adding distilled water to the mark. Then made a series of standard solutions with a concentration of 0; 2; 4; 8 ppm. Stock of 100 ppm gallic acid stock as much as 0; 0.2; 0.4; 0.8 mL each was added with 0.8 mL folin reagent, placed in a 10 mL volumetric flask. Next 5% Na2CO3 was added to the boundary mark, so as to produce a standard solution with a concentration of 0; 2; 4; 8 ppm. Each solution was allowed to stand for 60 minutes, and its absorption was measured at the maximum wavelength. By channeling absorbance to concentration, a calibration curve can be obtained with the regression equation y = bx + a
Determination of Total Phenolic Compound
Determination of the total content of these phenolic compounds was carried out based on the Folin-Ciocalteu method. Coral Plants leaf extract was dissolved with water then filtered using filter paper, then the filtrate was pipetted 1.0 mL in a 10 mL volumetric flask and added with 0.8 mL folin reagent. After that the mixture was shaken. Next 5% Na2CO3 was added to the mark, so the total volume of the solution becomes 10 mL. The solution was allowed to stand for 60 minutes, and its absorption was measured at a maximum wavelength of 750 nm. Measurement were repeated 3 times. The concentration of the compound was determined by converting the absorbance of the sample to the calibration curve and calculated as total phenol content in units of percent (%).
Results and Discussion
Phytochemical Properties
Coral Plants leaves were thought to have a high alkaloid compound and a little bit flavonoid and phenol. Phytochemical screening test result showed that the methanol extract from the leaves of Coral Plants (Jatropha mulitifida Linn) contained several active compounds, namely alkaloid, flavonoid, tannin, total phenols and terpenoids. The leaves had the function as an anti microbial. Flavonoid was anti-inflammatory so they can reduce inflammation and help reduce pain, if bleeding or swelling occurs in the wound. Phenol had the ability to form complex compounds with proteins through hydrogen bonds, which can damage bacterial cell membranes.6
Total Phenol
Total phenol was set on the leaves to determine level of phenol. Phenol level can show the nature of active substances as antioxidant and antimicrobial. total phenol was quite high found in the leaves average of 165,20 ± 33 mg GAE / mL. This showed that Coral Plants leaves have the potential to reduce free radical (antioxidant) and inhibit microbial growth. Polyphenol derivatives were thought to be antioxidant compound that can stabilize free radical by completing the lack of electrons possessed by free radical, and inhibit the chain reaction of free radical formation. Polyphenol were component which responsible for antioxidant activity in fruits and vegetable8. Besides, total phenol can act as an antimirobial compound. Phenol compounds had the ability to form complex compound with protein through hydrogen bond, which can damage bacterial cell membrane.9
Table 1: Total Phenol of Coral Plants Leaf
|
No |
Total Phenol (mg GAE / mL)* |
|
1. |
164, 16 ± 32 |
|
2. |
165, 34 ± 21 |
|
3. |
166,11 ± 46 |
|
Average |
165,20 ± 33 |
Antimicrobial Activity of Coral Plants Leaf Methanol Extract
To determine the antimicrobial activity of methanol extract on the Coral Plants leaf, an antimicrobial test (antibacterial and antifungal test) was conducted using the disc method. Antibacterial activity test was carried out on 2 types of bacteria (E.coli and Basillus sp) which can be seen in table 2.
Table 2: Inhibitory Potency (Anti-bacterial) of Coral Plants Leaf
|
Sample |
Inhibitory Potency (mm)* |
|
|
E- Coli |
Bacillus sp |
|
|
Leaf |
11,6 |
12,3 |
|
Standard Amoxcillin |
9 |
9 |
*} Average of 3 replication
Table 2 showed the methanol extract of Coral Plants leaf can inhibit E.coli gram negative bacteria with strong inhibition (10-20 mm) exceeding Amoxicilin standard, while gram positive bacteria (Bacillus sp) can only be inhibited by Coral Plants leaf methanol extract by 12.3 mm . According to Davis Stout in Rita, the antimicrobial category is weak (<5mm), moderate (5-10mm), strong (10-20mm) and very strong (> 20mm). Coral Plants leaf is the most active part in inhibiting the growth of gram-positive and negative bacteria. This property has been utilized by the community to utilize Coral Plants leaves as a wound medicine.
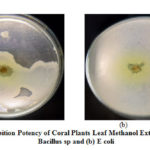 |
Figure 2: Inhibition Potency of Coral Plants Leaf Methanol Extract against |
Antifungal activity test was carried out on 3 types of fungi namely Aspergillus flavus, Tricodherma and Aspergillus niger by measuring the amount of inhibitory potency referring to the amount of clear zone formed around the disk. The result of antifungal test on the methanol extract of Coral Plants leaf can be seen in Table 3.
Table 3: Inhibitory Potency (Antifungal) of Coral Plants Leaf
|
Sample |
Inhibitory Potency *) |
||
|
Penicillium |
A. niger |
Tricoderma |
|
|
Leaf |
7 |
— |
4,5 |
|
Standard Ketokonazole |
13 |
18 |
13,5 |
*} Average of 3 replication
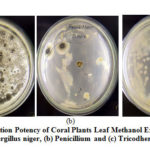 |
Figure 3: Inhibition Potency of Coral Plants Leaf Methanol Extract against |
Coral Plants leaves can inhibit the growth of Penicillium and Tricodherma fungi, but cannot inhibit the growth of Aspergillus niger fungi. Inhibition of fungal growth is in the medium category (5-10 mm) and lower than inhibition zone in the ketoconazole standard. Leaves can be used as treatment materials to heal wounds and as anti-inflammatory4because they contain flavonoid which are able to reduce inflammation and help reduce pain, if bleeding or swelling occurs in the wound. In addition, the presence of phenol compound has the ability to form complex compound with protein through hydrogen bonds, so that these compounds can synergize in healing wounds. Antimicrobial activity on Betadin leaf had been investigated by Liana and Putinah in 2016, which showed limited antibacterial activity from active fraction of Betadin leaf against Staphylococcus aureus.
Conclusion
Total phenol content was quite high in the leaf average of 165,20 ± 33 mg GAE / mL. This showed that Coral Plants leaves had the potential to reduce free radicals (antioxidants) and inhibit microbial growth. Antibacterial activity test result showed that the methanol extract of Coral Plants leaves can inhibit E.coli gram-negative bacteria with strong inhibition (10-20 mm) and gram-positive bacteria (Bacillus sp) by 12.3 mm. Based on the results of antifungal activity test, it was known that Coral Plants leaves can inhibit the growth of penicillium and Tricodherma fungi, but cannot inhibit the growth of Aspergillus niger fungi. The inhibition against Penicillium was higher (7 mm) compared to Tricodherma of 1.8 – 4.5 mm.
Acknowledges
The authors are grateful for the financial support received from Polytechnic AKA Bogor and for providing the required facilities to complete out this work.
Conflict of Interest
The authors disclosed no conflicts of interest related to this article.
References
- Abdurrahmat, A. S.; Gorontalo State University ENTOPI Journal , 2014, 9 (1),721-840.
- Apriyanti, M.;Yogyakarta: New Library Pres, 2010, 2
- Liana, Y. and Putinah ; Bina Husada Journal of Health, 2016, 11:479 – 485
- Nagaharika, Y., Kalyani, V., Rasheed S., and Karthikeyan R.; Journal of Acute Disease, 2 (2), 2013, 156–158.
- Sudaryono, A.; Media Medika Indonesia Journal, 2011, 45 (2)
- Iwan, J. and Nur, ; Bandung Medical Magazine, 2010, 42 (2) :77
- Coolborn, A.F. and Bolatito, B.; Journal of Natural Products. 2010, 3, 27-34
- Junior, M.R.M., Leite A.V., and Dragano N.R.V.,The Open Chemical Engineering Journal, 2010, 4: 51-60
- Balachandar, R., Saran, P.L., Ashok K.K., Ragavi A., and Gurumoorthy, P.; Journal of Chemical and Pharmaceutical Sciences, 2014, 1(2), 100–3
- Rita, W. S.; Journal of Chemistry, 2010,4: 20-26

This work is licensed under a Creative Commons Attribution 4.0 International License.









