Physicochemical Properties and Bactericidal Effects of Neutral Electrolyzed Water against Escherichia Coli and Salmonella Enterica on Ground Pork
Vinh Tien Nguyen , Thanh Tung Pham
, Thanh Tung Pham , Quang Duy Nguyen
, Quang Duy Nguyen  and Khanh Son Trinh*
and Khanh Son Trinh*
Faculty of Chemical and Food Technology, Ho Chi Minh City University of Technology and Education, No 1 Vo Van Ngan, Linh Chieu Ward, Thu Duc District, Ho Chi Minh City, Vietnam.
Corresponding Author E-mail: sontk@hcmute.edu.vn
DOI : http://dx.doi.org/10.13005/ojc/360315
Article Received on : 23 April 2020
Article Accepted on : 23 May 2020
Article Published : 28 May 2020
This study evaluated physicochemical and antibacterial properties of neutral electrolyzed water (NEW) produced by electrolyzing NaCl solutions. pH, total chlorine content (TC) and oxidation-reduction potential (ORP) of NEW increased to equilibrium values when increasing NaCl concentration (0.20% - 1.5%) and electrolysis time (0 – 240 min). The pH and ORP values increased sharply in the first 15-minute of the electrolysis and then was stabilized in the ranges of 8.5-9.5 and 400-500 mV, respectively. Increasing NaCl concentration did not change the stabilized values of pH and ORP, but significantly increased (p<0.05) TC. Furthermore, we studied antibacterial activity of NEW against Escherichia coli and Salmonella enterica in suspension and in ground pork. Interestingly, 085% NaCl NEW after 10-min electrolysis reduced 7 log CFU/mL of E. coli and 2 log CFU/mL of S. enterica. This resistance of S. enterica toward NEW was possibly due to its biofilm-forming ability.
KEYWORDS:Escherichia Coli; Ground Pork; Neutral Electrolyzed Water; Salmonella Enterica; Sodium Chloride.
Download this article as:| Copy the following to cite this article: Nguyen V. T, Pham T. T, Nguyen Q. D and Trinh K. S. Physicochemical Properties and Bactericidal Effects of Neutral Electrolyzed Water against Escherichia Coli and Salmonella Enterica on Ground Pork. Orient J Chem 2020;36(3). |
| Copy the following to cite this URL: Nguyen V. T, Pham T. T, Nguyen Q. D and Trinh K. S. Physicochemical Properties and Bactericidal Effects of Neutral Electrolyzed Water against Escherichia Coli and Salmonella Enterica on Ground Pork. Orient J Chem 2020;36(3). Available from: https://bit.ly/2XD20lv |
Introduction
Microbial foodborne intoxication is one of the major concerns in the food industry today. Electrolyzed water (EW) is a product with many potential applications, primarily in terms of eliminating microorganisms, for the food industry in many countries. EW is made by electrolysis of solutions of NaCl, MgCl2, KCl or HCl1. Depending on the electrolyte used for electrolysis, acidic, basic or neutral electrolyzed water can be obtained. Acidic electrolyzed water has been used to treat different bacteria such as Escherichia coli, Salmonella enteritidis, Salmonella typhimurium, Listeria monocytogenes, etc. with an inactivation up to 6.0 log CFU/mL of bacteria. The main disadvantage of acidic neutralized water is the gradual evaporation of Cl2 at low pH, resulting in a reduction of bactericidal ability over time. In addition, evaporated Cl2 gas adversely affects human health and pollutes the environment2. Moreover, low pH of electrolyzed water can cause equipment corrosion, and thus limiting its application range3.
Neutral electrolyzed water (NEW) can inactivate many microorganisms without affecting the sensory quality of food4,5. NEW is generated by electrolyzing solution of a chloride salt in a system without a diaphragm. In the anodic compartment, products such as H+, H2O2, O3, O2, Cl2, HClO, ClO3–, etc., are formed, while OH–, H2, NaOH, etc., are formed in the cathode compartment6. With careful control of the electrolysis conditions, NEW can be used for microbial treatment without deteriorating food quality7,8. The purpose of this study was to evaluate the effects of NaCl concentration on physicochemical properties of NEW and the ability to eliminate Escherichia coli and Salmonella enterica, two main pathogens causing food poisoning as well as common intestinal diseases in food. To the best of our knowledge, there was no study about using NEW to disinfect ground pork, which has large surface area and complex surface morphology.
Materials and Methods
Materials
Escherichia coli (VTCC-B-482) and Salmonella enterica (VTCC-B-480) bacterial strains used in this study were purchased from the Institute of Microbiology and Biotechnology (Vietnam National University, Hanoi). The bacteria were cultured in Nutrient Broth medium with continuous shaking (250 rpm) at 37oC for 24 h. Bacterial suspensions were diluted to 108-109 CFU/mL (8-9 log CFU/mL) to be used in this study.
The meat used was fresh ground pork purchased in a local supermarket. The pork was tested to ensure that it complied with the EUR-Lex standard (88/657/EEC) about meat for direct human consumption or for industry (Table 1).
Table 1: Microbiological criteria for fresh pork meat
|
Criteria |
Satisfactory |
|
Total viable count, CFU/g |
<105* |
|
Coliform, CFU/g |
<102 |
|
E. coli, CFU/g |
<102 |
|
Staphylococcus aureus, CFU/g |
<102 |
|
Clostridium perfringens, CFU/g |
<102 |
|
Salmonella, CFU/25g |
Not detected |
* <106.for ground pork
Production of Neutral Electrolyzed Water (NEW)
Sodium chloride solutions (0.2, 0.5, 0.85 and 1.5%, w/v) were sterilized at 121oC for 15 minutes. The sterilized solutions were cooled to room temperature and then poured into the electrolysis tank (Figure 1). The electrodes were two titanium plates (10 cm × 10 cm) coated with mixed metal oxides, including RuO2, IrO2 and TiO2, to prevent electrochemical corrosion. Electrolysis of the NaCl solutions was conducted with a controlled current of 3A and a voltage of 20 V using a DC power supply (CPS – 3205E, China).
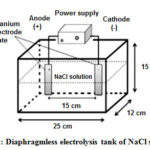 |
Figure 1: Diaphragmless electrolysis tank of NaCl solutions. |
Total Chlorine (TC)
The pH and the oxidation-reduction potential (ORP) of the electrolyzed water were measured in situ using a pH meter (SevenEasy, Switzerland) and an ORP tester (KKmoon Pen-Type ORP Meter, China)8. TC of the electrolyzed water was determined based on ISO 7393-3:1990 titration method9. Briefly, an aliquot of the electrolyzed water was mixed with 10.0 mL of a standardized 0.015 M Na2S2O3 solution. Afterwards, 1 g of KI, 2 mL of 0.87 M H3PO4 solution and 1 mL of 0.5% (w/v) starch solution were successively added. The solution was then titrated with a standard KIO3 solution until the appearance of a blue colour, which remained for at least 30 s.
Antibacterial Activity of NEW against E. Coli and S. Enterica on Petri Dishes
An E. coli or S. enterica suspension (0.1 mL) was mixed with NEW (0.9 mL) under stirring for 5 min at room temperature. To determine the concentration of survived bacteria, the mixture was then serially diluted by a 0.1% peptone solution and then spread on Nutrient Broth (NB) agar Petri dishes. The Petri dishes were subsequently kept at 37 oC for 24 h before the number of formed colonies on each dish was counted. The survived bacterial concentration was transformed to log CFU/mL of bacterial suspension. To conduct a negative control experiment, a phosphate buffer saline solution (0.9 mL, pH 9.0), instead of NEW, was mixed with the bacterial suspension. After 5 min of stirring, the concentration of survived bacteria was determined as aforementioned10.
Antibacterial Activity of NEW against E. Coli and S. Enterica in Contaminated Pork
To contaminate the pork, fresh pork (25g) was mixed thoroughly with 5 mL of a bacterial suspension of E. coli or S. enterica and then incubated at 37 oC for 24 h. The contaminated pork was then put in a sealed flask for 1 h at 4 oC for stabilization. To test the antibacterial effect of NEW on the contaminated pork, peptone (1%, 100 mL) and NEW (900 mL) were added to the flask. The flask was then shaken continuously for 5 minutes at room temperature (~30oC). After that, the concentration of survived bacteria was determined as in the method above11, 12. The negative control experiment was conducted in the same procedure with a phosphate buffer saline solution (0.9 mL, pH 9.0) replacing the NEW.
Statistical Analysis
All the antibacterial experiments were carried out in triplicate. Statistical analyses, including ANOVA and Tukey’s test (p <0.05) were conducted with SPSS statistical program package (version 20.0, USA).
Results and Discussion
Physicochemical Properties of NEW During Electrolysis
During diaphragmless electrolysis of NaCl solutions, the following main reactions take place8:
Anode: 2Cl–(aq) → Cl2(g)+ 2e (1)
Cathode: 2H2O (l) + 2e → H2 (g) + 2OH–(aq) (2)
Under stirring conditions and without diaphragm:
Cl2(g) + 2OH–(aq) ↔ 2ClO–(aq)+ H2O(l) (3)
The DC current transferred through the electrolyte solution resulted in the Ohmic heating effect that increased the temperature of the NaCl solutions (Figure 2). Moreover, the exothermic chemical reaction (3) was another reason for the temperature rise during electrolysis13. Higher concentrations of NaCl increased the electrical current and the extent of reaction (3), hence resulting in a higher temperature of the solution. However, the generated heat was exchanged with the environment, thus keeping a maximum temperature of about 45 oC after 240 min. From a practical point of view, it should be noted that high temperatures can lead to low solubility of chlorine gas, thus reducing TC values and the antibacterial effects of the NEW8, 12. This implies the necessity of a cooling mechanism when using this electrolytic technology to produce NEW.
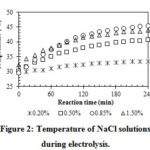 |
Figure 2: Temperature of NaCl solutions during electrolysis. |
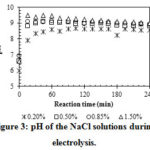 |
Figure 3: pH of the NaCl solutions during electrolysis. |
Another important property of the electrolyzed water that can affect its point-of-use applicability is the pH. Too high or too low values of pH can result in corrosion and deteriorate the food quality. In the range of NaCl concentration from 0.2 to 1.5 % (w/v), the pH values of the electrolyte solutions increased rapidly and reached their equilibrium value (from 8.4 to 9.0) after approximately 60 min of electrolysis (Figure 3). The pH values were higher than 7.0 due to the presence of hypochlorite ions, which are weakly basic anions. These slightly basic pH values are acceptable in the food industry and can be considered newtral8. There are no significant differences between the pH values of the electrolyzed water with NaCl concentrations of 0.5% and higher13, 14.
TC and ORP are important physicochemical properties of NEW that affect its antibacterial ability and technological applications. Their values should be high enough to ensure high antibacterial effects, but too high values of them can deteriorate the sensory quality of food and corrode equipment in contact8. Therefore, the values of TC, ORP and pH should be monitored and controlled during and after the electrolysis.
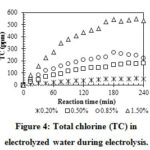 |
Figure 4: Total chlorine (TC) in electrolyzed water during electrolysis. |
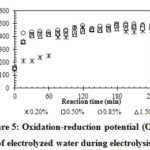 |
Figure 5: Oxidation-reduction potential (ORP) of electrolyzed water during electrolysis. |
In general, NaCl concentration has a significant effect on properties of EW, especially the total chlorine due to the formation of oxidizing agents (ClO– and Cl2) in reactions (2) and (3)14, 15, 16. The TC increased gradually with electrolysis time and was proportional to NaCl concentration (Figure 4). After 180 min, the TC values stopped increasing (1.5% NaCl) or even decreased (0.85% NaCl) due to the temperature increase in the NaCl solutions during electrolysis (Figure 2), which caused partial evaporation of Cl2 8.
Figure 5 shows that increasing the NaCl concentration from 0.2% to 0.5% resulted in a significant increase in the ORP during the first 60 min. However, no significant difference between the ORP values was observed at longer electrolysis times for all investigated NaCl concentrations. The chlorine compounds (Cl2, and OCl–) with high oxidizing ability are the reason for the increase in ORP values6, 13. Previous studies showed that ORP and TC value are highly correlated with the ability to kill microorganisms of antiseptic solutions6, 8, 17.
Antibacterial Activity of NEW against E. Coli and S. Enterica Suspensions
In this study, the NEW from 0.85% NaCl solution was chosen to test the antibacterial effects because this concentration is close to that of commercial physiological saline. Figure 6 demonstrates the high antibacterial effects of this NEW against E.coli in a suspension. After 10 min of electrolysis, the 0.85% NaCl solution containing approximately 30 ppm of TC reduced 9.23-log CFU/mL of E. coli. At the same time, S. enterica showed significant resistance toward NEW. Only 1.55-log CFU/mL reduction was observed for S. enterica, which corresponded to a 97% reduction in the bacterial concentration. Increasing electrolysis time to 20 min increased antibacterial effect to 2.42-log CFU/mL (99.6%) reduction of S. enterica.
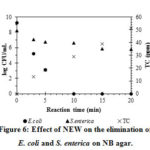 |
Figure 6: Effect of NEW on the elimination of E. coli and S. enterica on NB agar. |
 |
Figure 7: Biofilm of S. enterica on NB agar. |
The pH and ORP values of NEW obtained from 0-20 min of electrolysis were not significantly different (Figures 3 and 5). However, the TC value increased sharply during this period of electrolysis. Thus, we suppose that TC has a direct impact on the antibacterial effect of NEW. This result is in accordance with a previous study, in which hypochlorous acid and hypochlorite ions were the most active chlorine compounds in killing bacterial cells. The inactivation mechanism of these compounds was oxidation of sulfhydryl groups in important enzymes in the bacterial carbohydrate metabolism18.
Abadias19 showed that NEW (48 ppm of TC) could reduce more than 5.4-log CFU/mL of E. coli O157:H7 and Salmonella chloleraesuis. Moreover, Issa-Zacharia11 using slightly acidic and strong acidic electrolyzed waters (about 50 ppm of TC) reduced 5- to 6-log CFU/mL of E. coli and Salmonella ssp. Thus, the inactivation effectiveness of NEW in this study against E. coli was quite similar to previous studies. However, for S. enterica, the inhibition effects this study was quite lower than that of previous ones. A possible explanation was that the ability to create biofilm (Figure 7) at high bacterial density has helped S. enterica to resist better the effects of EW than E. coli20. This biofilm, which is composed of polymer layers called glycocalyx, after forming can encapsulate the bacterial cells and create a protective surface that allows Samonella spp. to survive in harsh environments, including disinfecting and gene-modification agents21, 22, 23, 24. Vestby21 suggested that the bactericidal hypochlorite ions (ClO–) cannot get in contact with bacterial cell walls due to the blocking mechanism of organic layers in this biofilm.
Antibacterial Activity of NEW against E. Coli and S. Enterica in Contaminated Pork
Ground meat is easy to be contaminated and hard to be disinfected due to its large surface area. However, after treatment with NEW, the amount of E. coli and S. enterica as well as the aerobic microorganisms available on pork were significantly reduced (Table 2).
Table 2: Bacteridal activity of NEW at various electrolysis times against E. coli and S. enterica in contaminated pork
|
Electrolysis time (min) |
TC (ppm) |
Total viable count in contaminated pork (log CFU/g) |
|
|
E. coli |
S. enterica |
||
|
0 |
0.00 ± 0.01a |
7.94 ± 0.10a |
7.54 ± 0.02a |
|
3 |
13.12 ± 0.00b |
6.65 ± 0.20b |
6.60 ± 0.07b |
|
5 |
18.63 ± 0.01c |
6.08 ± 0.06c |
5.82 ± 0.09c |
|
10 |
29.14 ± 0.11d |
ND |
5.80 ± 0.15c |
|
15 |
38.80 ± 0.20e |
ND |
5.78 ± 0.21c |
|
20 |
51.00 ± 0.59f |
ND |
5.77 ± 0.16c |
Results are expressed as mean ± standard deviation (n = 3). Different letters a, b, c in the same column indicate significantly different means (p<0.05). ND – Not detected.
Figure 6 and Table 2 show that NEW after 10 min of electrolysis can completely eliminate E. coli, both in suspensions and in contaminated ground pork. The antibacterial effects of NEW on ground pork was lower than that in suspension, because the ground pork with complex surface morphology acts as a physical barrier inhibiting the bactericidal effects of NEW25. In addition, Mahmound26 observed only 2-log CFU/g reduction in total bacteria on muscle meat after washing with acidic electrolyzed water (AEW) with 0.9% NaCl after 15 min of electrolysis. The difference in the antibacterial effects in the study of Mahmound26 with that in our study (7.94-log CFU/g reduction) may be due to the difference in properties of acidic and neutral electrolyzed waters: ORP = 1137 mV, pH 2.2, 40.8 ppm TC chlorine in Mahmound study26 and those in the present study ORP = 425 mV, pH: 9.09, 29.14 ppm TC. Although AEW solution with higher ORP can have better higher antibacterial effects, but some studies showed this effect decreased markedly with EW with pH lower than 2.73, 27. This can be explained from a chemical point of view: an acidic pH shift to equilibrium (3) to the left side, hence producing Cl2(g) in the form of tiny bubbles. The bubbles can escape easily from solution and has less contact areas with the bacteria cells than the soluble form of ClO–.
In this study, NEW after 10 min of electrolysis (29.14 ppm of TC) can completely inactivate 7.94-log CFU/g of E. coli on the ground pork sample. Because the maximum allowable level of E. coli contamination in fresh meat was 102 CFU/g and 106 for aerobic bacteria (according to Council Directive 88/657/EEC), NEW that can reduce from 5- to 6-log CFU/g can be used in the food industry16.
NEW after 20 min of electrolysis reduced only 1.77-log CFU/g of S. enterica (Table 2). Therefore, the treatment of S. enterica and bacteria available on pork meat by NEW has limited applicability. As mentioned above, the difference in results between the treatments of E. coli and S. enterica was due to the ability of S. enterica to create biofilms28. Salmonella is a Gram-negative bacterial family with a thick cell membrane29 and a biofilm mechanism that envelops even neighbour cells to resist antimicrobial agents28. According to Habimana30 and Corcoran31, when other aerobic bacteria are present together with S. enterica in the environment, especially Pseudomonas, Staphylococcus and Pantoea, etc., they promote S. enterica to create denser biofilms that enveloped the other bacteria, hence protect them from disinfecting agents. Therefore, it could be the reason for reducing NEWs antibacterial ability against S. enterica and other bacteria present on the contaminated pork.
Although the results in the laboratory show that NEW did not completely inactivate 7.54-log CFU/g of S. enterica in contaminated pork, the NEW still can find applications in sterilizing meat in the food industry. In reality, no consumable food is allowed to be contaminated with Salmonella at such a high density. In addition, biofilms from Salmonella form only when bacterial density is higher than 105 CFU/g32. Therefore, it is feasible to apply NEW in the disinfection of S. enterica in food, agricultural and medical fields.
Conclusion
This study showed that NEW can be used to disinfect meat contaminated aerobic bacteria, such as E. coli and S. enterica. Increasing NaCl concentration and/or electrolysis time increases physicochemical properties (temperature, pH, ORP, TC) and antibacterial activity of NEW. NEW from 0.85% NaCl solution and 10 min of electrolysis showed applicable results in disinfecting ability on ground meat. S. enterica at high density showed relatively high resistance toward NEW. This study suggests further investigations on the applicabilities of NEW on food contaminated with S. enterica at various extents.
Acknowledgment
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare no conflict of interest regarding the publication of this article.
References
- Rahman, S. M. E.; Wang, J.; Oh, D. H. Synergistic effect of low concentration electrolyzed water and calcium lactate to ensure microbial safety, shelf life and sensory quality of fresh pork. Food control. 2013, 30(1), 176-183.
- Len, S. V.; Hung, Y. C.; Erickson, M.; Kim, C. Ultraviolet spectrophotometric characterization and bactericidal properties of electrolyzed oxidizing water as influenced by amperage and pH. Journal of food protection. 2000, 63(11), 1534-1537.
- Guentzel, J. L.; Lam, K. L.; Callan, M. A.; Emmons, S. A.; Dunham, V. L. Reduction of bacteria on spinach, lettuce, and surfaces in food service areas using neutral electrolyzed oxidizing water. Food microbiology. 2008, 25(1), 36-41.
- Deza, M. A.; Araujo, M.; Garrido, M. J. Inactivation of Escherichia coli O157: H7, Salmonella enteritidis and Listeria monocytogenes on the surface of tomatoes by neutral electrolyzed water. Letters in applied microbiology. 2003, 37(6), 482-487.
- Cui, X.; Shang, Y.; Shi, Z.; Xin, H.; Cao, W. Physicochemical properties and bactericidal efficiency of neutral and acidic electrolyzed water under different storage conditions. Journal of Food Engineering. 2009, 91(4), 582-586.
- Chuang, C. Y.; Yang, S.; Chang, M. Y.; Huang, H. C.; Luo, C. H.; Hung, P. C.; Fang, W. Inactivation efficiency to Bacillus subtilis and Escherichia coli bacterial aerosols of spraying neutral electrolyzed water. Journal of the Air & Waste Management Association. 2013, 63(12), 1447-1456.
- Oh, D. H.; Marshall, D. L. Antimicrobial activity of ethanol, glycerol monolaurate or lactic acid against Listeria monocytogenes. International journal of food microbiology. 1993, 20(4), 239-246.
- Hricova, D.; Stephan, R.; Zweifel, C. Electrolyzed water and its application in the food industry. Journal of food protection. 2008, 71(9), 1934-1947.
- ISO 7393-3:1990. Water quality — Determination of free chlorine and total chlorine — Part 3: Iodometric titration method for the determination of total chlorine. International Organization for Standardization. 1990. https://www.iso.org/standard/14108.htmL
- Sanders, E. R. Aseptic laboratory techniques: plating methods. Journal of Visualized Experiments. 2012, (63), e3064.
- Issa-Zacharia, A.; Kamitani, Y.; Tiisekwa, A.; Morita, K.; Iwasaki, K. In vitro inactivation of Escherichia coli, Staphylococcus aureus and Salmonella spp. using slightly acidic electrolyzed water. Journal of bioscience and bioengineering. 2010, 110(3), 308-313.
- Nan, S., Li.; Y., Li, B.; Wang, C.; Cui, X.; Cao, W. Effect of slightly acidic electrolyzed water for inactivating Escherichia coli O157: H7 and Staphylococcus aureus analyzed by transmission electron microscopy. Journal of food protection. 2010, 73(12), 2211-2216.
- Hsu, S. Y. Effects of flow rate, temperature and salt concentration on chemical and physical properties of electrolyzed oxidizing water. Journal of Food Engineering. 2005, 66(2), 171-176.
- Al-haq, M. I.; Sugiyama, J.; Isobe, S. Applications of electrolyzed water in agriculture & food industries. Food Science and Technology Research. 2005, 11(2), 135-150.
- Kiura, H.; Sano, K.; Morimatsu, S.; Nakano, T.; Morita, C.; Yamaguchi, M.; Katsuoka, Y. Bactericidal activity of electrolyzed acid water from solution containing sodium chloride at low concentration, in comparison with that at high concentration. Journal of Microbiological methods. 2002, 49(3), 285-293.
- Ovissipour, M.; Al-Qadiri, H. M.; Sablani, S. S.; Govindan, B. N.; Al-Alami, N.; Rasco, B. Efficacy of acidic and alkaline electrolyzed water for inactivating Escherichia coli O104: H4, Listeria monocytogenes, Campylobacter jejuni, Aeromonas hydrophila, and Vibrio parahaemolyticus in cell suspensions. Food Control. 2015, 53, 117-123.
- Kim, C.; Hung, Y. C.; Brackett, R. E. Roles of oxidation–reduction potential in electrolyzed oxidizing and chemically modified water for the inactivation of food-related pathogens. Journal of food protection. 2000, 63(1), 19-24.
- Park, H.; Hung, Y. C.; Chung, D. Effects of chlorine and pH on efficacy of electrolyzed water for inactivating Escherichia coli O157: H7 and Listeria monocytogenes. International journal of food microbiology. 2004, 91(1), 13-18.
- Abadias, M.; Usall, J.; Oliveira, M.; Alegre, I.; Viñas, I. Efficacy of neutral electrolyzed water (NEW) for reducing microbial contamination on minimally-processed vegetables. International journal of food microbiology. 2008, 123(1-2), 151-158.
- Hall-Stoodley, L.; Costerton, J. W.; Stoodley, P. Bacterial biofilms: from the natural environment to infectious diseases. Nature reviews microbiology. 2004, 2(2), 95-108.
- Vestby, L. K.; Møretrø, T.; Langsrud, S.; Heir, E.; Nesse, L. L. Biofilm forming abilities of Salmonellaare correlated with persistence in fish meal-and feed factories. BMC veterinary research. 2009, 5(1), 20.
- Brown, M. R. W.; Gilbert, P. Sensitivity of biofilms to antimicrobial agents. Journal of Applied Bacteriology. 1993, 74, 87S-97S.
- De Beer, D.; Srinivasan, R.; Stewart, P. S. Direct measurement of chlorine penetration into biofilms during disinfection. Environ. Microbiol. 1994,60(12), 4339-4344.
- Donlan, R. M.; Costerton, J. W. Biofilms: survival mechanisms of clinically relevant microorganisms. Clinical microbiology reviews. 2002, 15(2), 167-193.
- Adams M.R., Moss M.O. Food Microbiology. 2nd The Royal Society of Chemistry. 2005.
- Mahmoud, B. S. M.; Yamazaki, K.; Miyashita, K.; Il‐Shik, S.; Dong‐Suk, C.; Suzuki, T. Decontamination effect of electrolysed NaCl solutions on carp. Letters in applied microbiology. 2004, 39(2), 169-173.
- Ozer, N. P.; Demirci, A. Electrolyzed oxidizing water treatment for decontamination of raw salmon inoculated with Escherichia coli O157: H7 and Listeria monocytogenes Scott A and response surface modeling. Journal of Food Engineering. 2006, 72(3), 234-241.
- Stepanović, S.; Ćirković, I.; Mijač, V.; Švabić-Vlahović, M. Influence of the incubation temperature, atmosphere and dynamic conditions on biofilm formation by Salmonella spp. Food Microbiology. 2003, 20(3), 339-343.
- Limoli, D. H.; Jones, C. J.; Wozniak, D. J. Bacterial extracellular polysaccharides in biofilm formation and function. Microbial Biofilms. 2015, 223-247.
- Habimana, O.; Møretrø, T.; Langsrud, S.; Vestby, L. K.; Nesse, L. L.; Heir, E. Micro ecosystems from feed industry surfaces: a survival and biofilm study of Salmonella versus host resident flora strains. BMC veterinary research. 2010, 6(1), 48.
- Corcoran, M.; Morris, D.; De Lappe, N.; O’connor, J.; Lalor, P.; Dockery, P.; Cormican, M. Commonly used disinfectants fail to eradicate Salmonella enterica biofilms from food contact surface materials. Environ. Microbiol. 2014, 80(4), 1507-1514.
- Rodrigues, D.; Teixeira, P.; Oliveira, R.; Azeredo, J. Salmonella enterica Enteritidis biofilm formation and viability on regular and triclosan-impregnated bench cover materials. Journal of food protection. 2011, 74(1), 32-37.

This work is licensed under a Creative Commons Attribution 4.0 International License.









