In Vitro Antimicrobial and in Vivo Molluscicidal Potentialities of Fe(III), Co(II) and Ni(II) Complexes Incorporating Symmetrical Tetradentate Schiff Bases (N2O2)
Chemistry Department, Faculty of Science, Albaha University, Al-Baha- KSA
Corresponding Author E-mail: samizabin@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/360304
Article Received on : 23 Jan 2020
Article Accepted on : 08 Jun 2020
Article Published : 18 Jun 2020
This paper aimed at synthesizing symmetrical Schiff base ligands derived from the primary amine 1,2-bis(2-aminophenylthio)ethane condensed with 7-formyl-8-hydroxyquinoline and 2-hydroxy-1-naphthaldehyde. The synthesised ligands were used for preparing metal complexes with the iron triad metals. The symmetrical tetradentate (N2O2) Schiff base ligands SL1 and SL2 forms mononuclear complexes with square planar geometry in case of Co(II) and Ni(II) complexes, while an octahedral geometry was obtained for the Fe(III) complexes. The molar conductance quantification showed that the complexes were non-ionic. In vitro antimicrobial potential examination of the free ligands indicated weak activity and the corresponding complexes showed enhanced activity. Moreover, the in vivo molluscicidal potential of the tested chemicals showed good activity against the tested land snails. Ligands showed activity at concentration of 1000 ppm while their metal complexes show activity at concentration of 500 ppm. The highest activity (LC50) of complexes was shown at concentration of 5000 ppm.
KEYWORDS:Antimicrobial Potential; Iron Triad Metals; Molluscicidal Activity; Symmetrical Schiff Bases; 1,2-Bis(2-Aminphenythio)Ethane
Download this article as:| Copy the following to cite this article: Alosaimi A. M, Mannoubi I. El, Zabin S. A. In Vitro Antimicrobial and in Vivo Molluscicidal Potentialities of Fe(III), Co(II) and Ni(II) Complexes Incorporating Symmetrical Tetradentate Schiff Bases (N2O2). Orient J Chem 2020;36(3). |
| Copy the following to cite this URL: Alosaimi A. M, Mannoubi I. El, Zabin S. A. In Vitro Antimicrobial and in Vivo Molluscicidal Potentialities of Fe(III), Co(II) and Ni(II) Complexes Incorporating Symmetrical Tetradentate Schiff Bases (N2O2). Orient J Chem 2020;36(3). Available from: https://bit.ly/3djzyLD |
Introduction
More than one hundred and fifty years have passed since the discovery of Schiff base ligands (1864) by the German scientist Hugo Schiff. These chemical compounds are well known and characterized by the presence of the distinguished azomethine functional group (>CH=N-). The presence of diverse and huge primary amine and carbonyl compounds with different substituents facilitate the preparation of these compounds with numerous structural designs. The Schiff bases as multi-dentate ligands provides excellent and diverse coordination sites that readily coordinate and strongly bind with different metal ions with different oxidation states forming stable metal coordination compounds. The Schiff bases and their metal complexes are reported to have vast biological applications and the presence of the functional azomethine group is reported as the critical factor in their biological activities [1-3]. Because of this moiety researchers have extensively investigated and still looking for developing new efficacious bioactive chemicals with azomethine scaffold.
Investigating symmetrical and asymmetrical systems of ligands for chelation with metal ions and assessing their biological application represents an important issue in coordination chemistry. In the last few years, different transition metal complexes incorporating symmetrical and asymmetrical Schiff bases have been reported in the literature [4-6]. The interest in designing and preparation of symmetrical and unsymmetrical Schiff bases is due to their ease of preparation using condensation reactions between different mono carbonyl compounds with different primary diamine compounds or mono primary amine compounds with dicarbonyl compounds.
In the light of the literature survey, we are encouraged to tailor new Schiff base ligands that contain several functional groups in one structure, which is expected to have some biological importance. The targeted ligands were synthesized using the primary amine1,2-bis(2-aminophenylthio)ethane that were condensed with two aldehydes 7-formyl-8-hydroxyquinoline and 2-hydroxy-1-naphthaldehyde. The synthesized symmetrical Schiff bases were used to synthesize metal complexes by reaction with iron triad metal (Fe, Co, Ni) salts in 1:1 molar ratio. All newly prepared compounds were characterized and structures were elucidated utilizing physicochemical measurements and different spectroscopic techniques. The synthesized compounds were investigated in vitro for their antimicrobial potentials. In addition, they were examined in vivo for their molluscicidal activity against terrestrial snails.
Experimental
All the chemical materials were of reagent grade and used in experimentation as purchased from market. The precursor chemicals, primary amine 1,2-bis(2-aminphenythio)ethane and 7-fromyl-8-hydroxy quinolone were prepared in the laboratory as given below.
Synthesis of 1,2-bis(2-Aminphenythio)Ethane
The precursor primary amine 1,2-bis(2-aminphenythio)ethane was synthesized following the reported method in the literature [7]. 100 mmol of 2-aminothiophenol and 100 mmol NaOH were mixed in 60 mL deionized water and refluxed for nearly half an hour. After cooling the mixture to 60 °C, 4.948 g of 1,2-dichloroethane was added drop-wise within half an hour and continue the refluxing for nearly two hours. After completion of the reaction, it was cooled to room temperature and the obtained product was extracted with chloroform. Chloroform solvent was evaporated to dryness, washed with ethoxyethane solvent and the obtained compound was dried over anhydrous magnesium sulfate (MgSO4) in a desiccator at room temperature. The obtained yellow solid product was stable at room temperature and the yield obtained was in good quantity 85%. The measured melting point was 68.9 °C. The reaction scheme (1) is shown below.
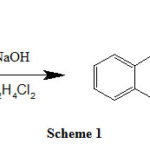 |
Scheme 1 |
Synthesis of 7-Formyl-8-Quinolinol
The aldehydic quinoline compound 7-Formyl-8-quinolinol was synthesized from 8-hydroxyquinoline following reported method [8-9]. A mixture of 1.45 g (100 mmol) of 8-Quinolinol, 90 mL of 15% NaOH solution and 8.5 mL of CHCl3 solvent was heated using water bath and refluxed for a period of four hours. After cooling, the pH of the reaction mixture was set at 5.5–5.8 using a solution of hydrochloric acid with molarity of 0.1M. The separated product obtained was light brown in color product that was collected by filtration and then purified by recrystallization from 80% ethanol to get pale brown solid. The yield (87%) obtained was in good quantity. The melting point measured was 210 °C.
Preparation of the Ligand (SL1)
SL1 ligand was prepared by adding a solution of 7-formyl-8-hydroxy-quinoline (10 mmol, 1.73 g) in chloroform (20 mL) to a solution of 1,2-bis(2-aminophenylthio)ethane (5 mmol, 1.38 g) in pure ethanol (25 mL) during half an hour at 60 °C with continuous stirring. The mixture was refluxed for 8 hours at 85 °C with stirring. After cooling the obtained precipitate was filtered off and washed with ethyl alcohol, chloroform and finally with ethoxyethane. The solid chemical obtained was dried in open air at 25°C. The resulted light orange solid product was recrystallized with hot chloroform and ethanol (50:50) solvent mixture to get yield of 76.3% and the melting point was 274 °C. SL1 synthesis scheme (2) is illustrated bellow.
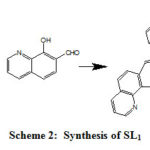 |
Scheme 2: Synthesis of SL1 |
Molecular formula (C34H26N4O2S2), m/z: 585.42[L–H]+ (Mol. Wt.: 586 g mol-1). Elemental analysis: Calculated (found) %C 69.61(70.12); %H 4.47(4.55); %N 9.56(9.76); %O 5.46(5.34); %S 10.91(10.96).
Preparation of the Ligand (SL2)
Similar to SL1 The ligand SL2ligand was prepared by adding a solution of 2-hydroxy-1-naphthaldehyde (20 mmol, 3.44 g) in absolute ethanol (40 mL) to a solution of 1,2-bis(2-aminophenylthio)ethane (10 mmol, 2.76 g) in absolute ethanol (20 mL) and refluxed for 8 hours at 80 °C with continuous stirring. The resulted yellow solid product was recrystallized with hot ethyl alcohol to get high yield of 90.21% and melting point was measured and found 245°C. SL2 ligand reaction scheme (3) is illustrated bellow.
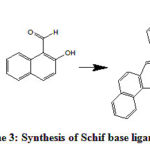 |
Scheme 3: Synthesis of Schif base ligand SL2 |
Molecular formula (C36H28N2O2S2), m/z: 584.42 [L–H]+ (Mol. Wt.: 584 g mol-1). Elemental analysis: Calculated (found) %C 69.61(70.00); %H 4.47(4.28); %N 9.56(9.67); %O 5.46(5.59); %S 10.91(11.16).
General Procedure for Preparation of Metal Complexes
The targeted metal complexes were prepared following the general reported method by the reaction between the hydrated metal salts of FeCl3.6H2O, CoCl2.6H2O and Ni(OCOCH3)2.4H2O with the Schiff base ligands [7, 10]. A mixture of the metal salt and the above-prepared Schiff bases were mixed in 1:1 molar ratio using ethanol as solvent and refluxed with stirring using hot stage magnetic stirrer for 4-5 hours until the reaction is completed. Then the isolated produced materials were separated and purified by recrystallization using hot dimethylformamide(DMF) solvent.
Physical Measurements and Spectroscopic Analysis
The obtained Schiff base ligands and metal complexes were analyzed and characterized by various physical and spectroscopic analytical techniques. The melting points were measured utilizing digital melting point apparatus model A9100 Electrothermal series. C, H, N, O and S percentage analysis were obtained using Thermo Fisher Scientific CHN/S/O analyzer instrument (Leco Model VTF-900 CHN-S-O 932 version 1.3x USA). Metal percentages were obtained using Inductive-coupled plasma (ICP) spectrophotometer (Thermo Scientific ICP-7000 plus Series ICP-OS). The mass spectral was recorded on Thermo Scientific-LCQ fleet ion trap mass spectrometer. FT-IR spectra obtained on Thermo Scientific Nicolet iS50 FT-IR spectrophotometer in the range 400-4000 cm-1 . The electronic absorption spectra in DMSO solvent were recorded on an Evolution 300 UV-visible double beam Spectrophotometer (ThermoFisher scientific company). 1H NMR spectra was recorded on Varian Mercury-400BB (400 MHz) spectrometer operating at 400 MHz. AP85 portable waterproof PH/conductivity meter was usedto measure the molar conductivities of the synthesized metal coordination compounds in DMSO or DMF at room temperature. Magnetic susceptibility of the prepared metal complexes was measured on Gouy’s Method Apparatus (Model No: HO-ED-EM-08)). Thermo-gravimetric analytical (TGA) measurements was performed on Shimadzu thermo-analyzer 50 in a dynamic nitrogen atmosphere (20 ml/min) at a heating rate of 10°C/min in the temperature range 25-1000°C and sample mass for each compound tested were 5 mg.
In Vitro Antibacterial and antifungal Activity Experimentation
In this investigation to test the antimicrobial potentials of the prepared compounds, the test experiments were performed at the laboratories of the Blood Bank- Clinical Microbiology Department – Al-Baha city. The antibacterial assay was performed against three types of Gram-positive and three Gram-negative bacterial strains. The Gram-positive bacterial strains used wereS. epidermidis (ATCC12228),S. aureus (ATCC 25923) and E. faecalis (ATCC 29212). The three Gram-negative bacterial strains wereP.aeruginosa (ATCC 27853), E. coli (ATCC25922) and P. mirabilis (ATCC 13376). The common pathogenic fungal strain C. Albicans (ATCC10231)was used for antifungal tests. The assessment experimental method followed was disk-diffusion agar test technique [11]. For experimental tests, stock solutions of the tested compounds were prepared by dissolving 0.01 g of each compound in 5 mL DMSO solvent. DMSO solvent was used as negative control. Amoxicillin antibacterial drug was used as reference for comparison. The effectiveness of each compound was quantified using zones of complete inhibition (in mm) after the incubation period. Each test was reduplicated and the mean values were registered.
In Vivo Molluscicidal Activity of the Prepared Compounds
The obtained complexes and their parent Schiff bases were examined for their molluscicidal potential following reported methods [6, 12]. Solutions of the tested compounds were prepared with concentrations ranging from (50ppm,100ppm, 500ppm, 1000ppm and 5000ppm) in DMSO solvent.
The snails (30 in number) used in this investigation were of the fresh water snails named Biomphalaria arabica (B. arabica). The snails were collected from wadis and near the water reservoirs at local Al-Baha city area. The collected snails were nursed for sometimes in the laboratory in order to habituate with laboratory conditions before evaluation tests. The tested snails were divided into groups and each group of 5 snails. The tested snails were kept at nearly 24 °C (i.e. room temperature) in plastic container with dimensions of (30 × 15 × 10 cm) containing some soil and fresh water and pH value was kept neutral at 7.0. The snails were fed with some green lettuce leaves and commercial fish food. Every two days we were usually replacing the water in the container.
The lethal concentration (LC50) of the tested chemicals was observed after twenty-four hours following the World Health Organization (WHO) guidelines [13]. Immersion method was used for this assessment and niclosamide drug was utilized as a control measure. Leitchfield and Wilcoxon dose effect were used as evaluation method14. Each experiment was reduplicated for each compound.
Results and Discussion
Preparation of Schiff Base Ligands
The Schiff base ligands designed in this project were synthesized following standard reported procedures as indicated in the previous section of materials and methods. For preparing the tailored symmetrical tetradentate Schiff base ligands, the primary amine 1,2-bis(2-aminphenythio)ethane was condensed with 7-formyl-8-hydroxyquinoline (SL1)and 2-hydroxy-1-naphthaldehyde (SL2). The stoichiometric reaction ratio of the primary amine to the aldehydic compound was (1:2). The synthesized Schiff bases were purified by recrystallization and were in good yields. They were colored; stable and most of them were soluble in hot ethyl alcohol, DMF, chloroform, and DMSO.
The isolated symmetrical Schiff base ligands were characterized and structures were confirmed before proceeding for the preparation of the metal complexes step. The elemental and mass spectral analysis were consistent with the theoretically computed elemental % and the proposed molecular formulae and hence confirm and prove the proposed structures of the symmetrical Schiff base ligands. The observed mass spectra for the ligands SL1 and SL2 were 585.42 and 584.42 corresponding to the molecular formulae C34H26N4O2S2, (M. Wt. =586) and C36H28N2O2S2, (M. Wt. =584) respectively.
Preparation of Metal Complexes
In this investigation, we have chosen the iron triad transition metals (i.e. iron, cobalt and nickel) for synthesis of the tailored metal coordination compounds. The iron triad metals were selected because they show similarity among themselves in physical and chemical properties and are adjacent to each other in the first series of the d-block elements (4d period in the periodic table).
The targeted metal complexes were synthesized using symmetrical tetradentate Schiff base ligands SL1 and SL2. These metal coordination compounds were prepared successfully according to the template method. The designed transition metal coordination compounds were prepared in the laboratory using 1:1 metal to ligand stoichiometric ratio.
The different metal complexes produced were non-hygroscopic colored amorphous solids, stable at room temperature. They were purified by recrystallization from hot DMF and DMSO solvents. The metal complexes obtained were soluble in hot DMF and DMSO solvents and the metal coordination compounds decomposed without melting > 300 °C. Table (1) below represents the molecular formulae, molecular weights, colors, and yields of the prepared coordination compounds.
Table 1: Physical properties of the prepared metal complexes
|
Metal Complex |
Mol. formula |
Mol. Wt. |
Mass Spectrum (m/z) |
Color |
Yield (%) |
|
[FeSL1 .2H2O] |
FeC34H28N4O4S2 |
676.00 |
677.14 |
Reddish brown |
76.50 |
|
[NiSL1] |
NiC34H24N4O2S2 |
642.83 |
641.33 |
Brown |
80.20 |
|
[CoSL1] |
CoC34H24N4O2S2 |
643.07 |
641.42 |
Brown |
84.56 |
|
[FeSL2 .2H2O] |
FeC36H30N2O4S2 |
674.01 |
675.24 |
Dark red |
87.49 |
|
[NiSL2] |
NiC36H26N2O2S2 |
640.83 |
641.42 |
Brown |
95.08 |
|
[CoSL2] |
CoC36H26N2O2S2 |
641.08 |
642.43 |
Brown |
82.4 |
Table 2: Elemental analysis of the Schiff base Complexes
|
Compound |
Elemental Analysis Calculated (found) |
|||||
|
% C |
% H |
% N |
% O |
% S |
% of metal |
|
|
[FeSL1 .H2O] |
60.35 (60.80) |
4.17 (4.28) |
8.29 (8.62) |
9.46 (9.74) |
9.46 (9.14) |
8.26 (8.47) |
|
[NiSL1] |
63.47 (63.22) |
3.76 (3.98) |
8.71 (8.60) |
4.98 (5.32) |
9.95 (10.18) |
9.13 (9.54) |
|
[CoSL1] |
63.45 (63.62) |
3.76 (4.10) |
8.71 (9.00) |
4.97 (4.52) |
9.94 (9.63) |
9.16 (9.42) |
|
[FeSL2 .H2O] |
64.09 (64.45) |
4.49 (4.70) |
4.16 (4.44) |
9.49 (9.40) |
9.49 (9.86) |
8.29 (8.69) |
|
[NiSL2] |
67.41 (67.08) |
4.09 (3.91) |
4.37 (4.47) |
4.99 (5.23) |
9.98 (9.57) |
9.16 (9.35) |
|
[CoSL2] |
67.39 (67.23) |
4.09 (4.25) |
4.37 (4.79) |
4.99 (5.31) |
9.97 (10.19) |
9.19 (9.37) |
The stoichiometry, formulation and geometry of the synthesized coordination compounds were characterized and proved using different physical and analytical methods. The suggested structures of the metal complex compounds were formed 1:1 metal to ligand ratio based on the elemental analysis as illustrated in table (2), and the mass spectral analysis table (1). The proposed structures for the synthesized Fe(III), Ni(II) and Co(II) complexes from the symmetrical tetradentate Schiff base ligands (N2O2) are depicted in scheme 4 in case of cobalt and nickel and scheme 5 for iron complexes. The ligands provide four donor atoms two nitrogen atoms of the azomethine groups, two oxygen atoms of the phenolic groups as coordination sites forming tetracoordinate monomeric Fe(III), Ni(II) and Co(II) complexes (schemes 4 and 5). The coordinated atoms of the ligand are symmetrically arranged around the metal ion. These complexes have the general formula [M(SL)] in case of cobalt and nickel, and the general formula for iron complexes is [M(SL).2H2O].
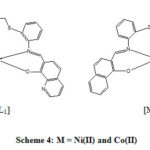 |
Scheme 4: M = Ni(II) and Co(II) |
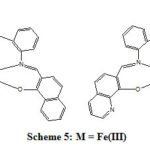 |
Scheme 5: M = Fe(III) |
The elemental analysis as illustrated in table 2 are consistent with the predicted structures of both types of complexes. Moreover, the synthesized metal complexes showed the ion molecular peaks (m/z) as shown in table (1), which were corresponding to the proposed molecular formula and in agreement with the calculated molecular weights of the metal complexes.
FT-IR Spectral Analysis
The IR spectrums of the prepared Schiff base compounds were recorded in order to confirm the formation of the designed ligands. Model peaks in the recorded IR spectrum were of good assist for characterizing and achieving this object. The characteristic wide band in the spectrum of the different prepared Schiff base ligands aroused in the range of 1553 -1560 cm-1 is assigned to the (>CH=N-) group mark the formation of the required Schiff base ligands [10]. The total absence of the band due to >C=Ogroup of the coupled aldehydes precursors supports the formation of the required Schiff bases. The wide bands appeared in the range of 3279.9 – 3420 cm-1 in the IR spectrum of the SL1 and SL2Schiff base ligands were allotted the phenolic (-OH) group stretching vibration f [15]. The bands at the range of 754.70 – 768.91 cm-1 can be designated C-S group [15].
The IR spectra of the metal complexes showed that the characteristic azomethine band was moved to another position in the spectra of the metal complexes (1504 – 1597.7 cm-1). This phenomenon proves the binding of N atom of the >CH=N- group to the metal ions [16]. This also was supported by the appearance of new medium band at 438.6 – 476.0 cm-1 due to the coordination bond (M-N) in the IR spectrum of the metal complexes.
The bands due to the stretching vibration of phenolic OH appeared in the spectrum of the Schiff base ligands were absent in the IR spectra of their metal complexes signifying the binding of the O atom of the -OH group with metal ion center. A medium band was observed in the IR spectrum of the complexes at the range of 501 – 537 cm-1 pointed to M-O bond [10, 17]. These observations are an evidence for the coordination of the symmetrical Schiff bases with the metal ions through the two nitrogen atoms of the azomethine groups and the two oxygen atoms of the phenolic groups and hence SL1 and SL2 ligands behave as tetradentate ligands.
In the IR spectrum of Fe(III) complexes a wide band was observed in the range of 3642 – 3780 cm-1 may be due to the coordination of H2O molecules to the iron metal.
UV–Visible Spectra and Magnetic Moment Measurements
The UV-visible spectral data of the ligands and their corresponding iron, Cobalt and nickel complexes were recorded in DMSO solution with a concentration of 1× 10-3M. The UV-visible absorption spectra of the free Schiff base ligands exhibit two bands in the range 253 – 285 nm and 325-370 nm assignable to the transitions π→π* of the benzene ring and n→π* transitions of the (>C=N-) group respectively [ 18-19].
The absorption spectra of the analyzed metal complexes of Co(II), Fe(III) and Ni(II) complexes have exhibited bands at lower intensities and longer wavelength compared to those of the free Schiff base ligands. This bathochromic shift (or red shift) may be due to the coordination of the N atom of the azomethine group and O atom of (-OH) group with the metal ion [20-21].
The absorption spectra of the coordination compounds display a weak band shoulder appeared in the range of 470-490 nm, which is attributed to the metal-ligand charge transfer (MLCT) transitions that expected for coordination compounds of low oxidation state metals[18]. This charge transfer band is a result of formation of the coordination bond between the metal ion and the ligands that causes variation in the electron distribution between the ligand and the metal ion. The d-d transitions were not observed since they are perturbed by the broad charge transfer transitions [22].
The discussion for each metal complexes are given below.
Co(II) Complexes
The UV-visible spectrum of the Co(II) complex compounds obtained from the symmetrical Schiff base ligands showed bands at 315-330 and 365-380 nm, which may be attributed to the intra-ligand absorption π→π* and n→π* transition of both conjugated and azomethine group respectively [22]. These transitions pointed out the formation of a square planar geometry for Co(II) complex compounds. This was supported by magnetic moment measurements for some cobalt complexes observed in the range of μeff = 1.94 – 2.15 B.M. These low-spin values of magnetic moment for cobalt complexes indicate paramagnetic behavior with one unpaired electron. Tetra-coordinate cobalt complexes with unpaired electrons are expected to have square planar geometry, which is more stable probably due to a combination of steric and electronic reasons [22-23].
Fe(III) Complexes
Fe (III) complex compounds obtained from the symmetrical Schiff base ligands (i.e. SL1 and SL2) exhibited bands in the range of 650-655, 585-590, and 545-555 which are characteristic of octahedral geometry around the Fe(III)ion [24-25]. Moreover, the high-spin magnetic moment value of 4.96-5.02 B.M. favors this suggestion [26-27]. In literature reported that hexa-coordinated d5-iron(III) complexes with Schiff base ligands are known to display a variety of magnetic behavior and Fe(III) complexes favor octahedral geometry [28].
Ni(II) Complexes
The UV-visible spectrum of Nickel (II) complex compounds obtained from the tetradentate symmetrical Schiff base ligands (SL1 and SL2) exhibited two bands in the range of 455-460 nm of the d-d transition may be assigned to 1A1g → 1B1g transitions and a low wavelength at 275 and 335 nm in the UV-visible spectrum. These bands are characteristic of the square planar geometry. Moreover, Nickel complexes of the tetradentate symmetrical Schiff bases at room temperature exhibited μeff values of diamagnetic behavior supporting the presence of square planar geometry around the Ni(II) ion [ 24, 29-30].
1H and 13C NMR Analysis
The 1H and 13C NMR Spectra analysis were done for the prepared ligands and their metal complexes and were noted in DMSO-d6 and CDCl3.
The sharp singlet at 8.39 ppm was visible in the spectrum of the three ligands because of the azomethine protons (CH=N) and hence assure the formation of the imine group. In metal complexes this signal suffers shifting to 8.02 – 8.27 ppm, suggesting that the azomethine group takes part in chelation [31].
Moreover, the signals of singlet at 11 ppm (SL1) and 13.2 ppm (SL2) confirmed the presence of phenolic -OH protons in the Schiff base ligands and were not observed in the metal Schiff base complexes [31-32]. The1H NMR spectrum of ligands SL1 and SL2 show signal peaks at 3.08 and 3.13 ppm is assigned to protons of methylene group attached to sulfur [33]. The multiplets in δ 6.92 – 7.29 ppm and 7.31 – 8.21 ppm regions that are specific to the protons of benzylidene ring groups. All of the protons were observed in their expected regions and numbers.
The 13C-NMR spectra of the ligands show peaks at range 109.51 -139.22 ppm and 118.49 – 129.78 ppm regions, which are assignable to the carbons of the aromatic rings. The HC=N carbon resonance is observed at 143.07 and 164.98 ppm in the spectrum of SL1 and SL2. This carbon was shifted to the downfield approximately 5 ppm relative to the free ligands confirming the transfer of one lone pair electron from nitrogen to metal and coordination of azomethine-N to metal [33].
TGA analysis
The simultaneous thermogravimetric analysis (TGA) and differential thermal analysis (DTA) of the prepared coordination compounds was investigated from the temperature of the surroundings to 1000°C in nitrogen atmosphere. This TGA and DTA analysis are carried out in order to support the proposed structures for the synthesized metal complexes. The correlation between the various decomposition steps of metal complexes with corresponding weight losses are argued according to the predicted formula of the metal complexes. The thermogram of the selected coordination compounds are depicted in Fig. (1-3).The effect of the heating rate on the thermal decomposition process notes are summarized in the following points:
The TG curve (Figure 1) of [CoSL2] complex first shows a slow decomposition from 140 to 320 °C, with 40.75% (calcd. 45.56%) mass loss. The broad exothermic ΔTmax = 250 °C in DTA curve referred to the removal of organic moiety corresponding to the half of ligand SL2. A sharp exothermic peak at 570 °C was shown in the DTA curve from 440 to 630 °C. The mass of final residue was calculated to be 27.01%, which is consistent with the theoretical value for the stable Co2O3, (28.31 %).
The TGA curve (Figure 2) of [FeSL2 .2H2O] complex first exhibited a mass loss of 5.07% (calcd. 5.05%) in the temperature range 120 – 210 °C with an endothermic DTA peak was observed in this region (ΔTmax = 178 °C) pointed out the removal of two molecules of coordinated water. The anhydrous coordination compound first exhibited decomposition from 220 to 300 °C, with 22.58 % (calcd. 22.42%) mass loss, a broad exothermic peak with ΔTmax = 250 °C in the DTA curve probably referred to the removal of organic moiety corresponding to the uncoordinated part of the ligand SL2 (C10H7O). The decomposition in the second step was observed between 420 to 560 °C, and a sharp exothermic peak in the DTA curve at 510 °C was shown. The calculated mass percentage (36.25%) of the final residue was consistent with that for the stable Fe3O4 (38.30 %).
The TG curve (Figure 3) of [NiSL2] complex first decomposition was exhibited in the temperature range 180 to 380 °C and the calculated mass loss percentage 45.58% consistent with the theoretically calculated percentage of 44.81% which is probable due to the removal of organic moiety corresponding to the half of ligand SL2. The broad exothermic peak at ΔTmax = 250 °C was observed in the DTA curve in this step. The second decomposition was exhibited in the temperature range from 440 to 690 °C and a sharp exothermic peak in the DTA curve was shown at 550 °C for this step. The calculated mass percentage of final residue was 25.78% which is consistent with the mass percentage (24.34 %) of the stable Ni2O3.
![Figure 1: (a) TG and (b) DTA curves of [CoSL2]](http://www.orientjchem.org/wp-content/uploads/2020/06/Vol36No3_Invit_Ama_Fig1-150x150.jpg) |
Figure 1: (a) TG and (b) DTA curves of [CoSL2] |
![Figure 2: (a) TG and (b) DTA curves of [FeSL2 .2H2O]](http://www.orientjchem.org/wp-content/uploads/2020/06/Vol36No3_Invit_Ama_Fig2-150x150.jpg) |
Figure 2: (a) TG and (b) DTA curves of [FeSL2 .2H2O] |
![Figure 3: (a) TG and (b) DTA curves of [NiSL2]](http://www.orientjchem.org/wp-content/uploads/2020/06/Vol36No3_Invit_Ama_Fig3-150x150.jpg) |
Figure 3: (a) TG and (b) DTA curves of [NiSL2] |
Molar Conductance Behavior
The molar conductance measurements of the prepared metal complexes were measured in DMSO solution of 10-3M solution in order to determine the ionic nature of the coordination compounds. The DMSO solvent was selected because all complexes are soluble in it at room temperature (22.4 °C). The molar conductance observation results (table 3) showed variety and low molar conductance values (1.10 – 64.3 S.cm2.mol-1) indicating non-electrolytic behavior (non-ionic nature) [34].
Table 3: The recorded molar conductivity of the prepared Complexes
|
Metal complex |
Molar Conductivity (S.cm2.mol-1) |
|
[FeSL1 .2H2O] |
49.15 |
|
[NiSL1] |
4.62 |
|
[CoSL1] |
45.35 |
|
[FeSL2 .2H2O] |
35.5 |
|
[NiSL2] |
1.10 |
|
[CoSL2] |
64.3 |
In vitro Anti-Microbial Assay
In this investigation, the prepared symmetrical Schiff base ligands along with their Fe(III), Ni(II) and Co(II) metal complexes were screened in vitro for their antimicrobial behavior against three Gram +ve and three Gram -ve bacterial strains and one fungal strain. The observations of the experimentation results are shown in table (4).
Table 4: Zones of complete inhibition of the ligands and their coordination compounds
|
|
Zone of inhibition (mm) |
||||||
|
Compound |
Gram-positive bacteria |
Gram-negative bacteria |
Fungus |
||||
|
S. epidermidis |
S. aureus |
E. faecalis |
P. aeruginosa |
E. Coli |
P. mirabilis |
C. Albicans |
|
|
SL1 |
5 |
7 |
9 |
R |
R |
R |
R |
|
SL2 |
6 |
9 |
8 |
R |
R |
R |
R |
|
[NiSL1] |
17 |
8 |
12 |
R |
R |
7 |
R |
|
[CoSL1] |
18 |
16 |
13 |
8 |
10 |
23 |
R |
|
[FeSL1 .2H2O] |
14 |
12 |
15 |
R |
R |
8 |
R |
|
[NiSL2] |
6 |
5 |
5 |
R |
R |
R |
R |
|
[CoSL2] |
19 |
16 |
20 |
21 |
8 |
21 |
R |
|
[FeSL2 .2H2O] |
12 |
7 |
8 |
R |
R |
22 |
R |
|
Standard Amoxicillin |
28 |
27 |
26 |
8 |
22 |
44 |
— |
** Key to interpretation: R = 0 = Resistant, less than 10 mm = inactive, 10–15 mm = weakly active, 15–25 mm = moderately active; more than 25 mm = highly active.
The examined Schiff base ligands (SL1 and SL2) showed low antibacterial activity towards the Gram-positive bacterial types with growth restrict diameters < 15 mm). The Gram-negative bacterial types exhibited resistance to the free ligand chemicals. Moreover, the prepared symmetrical ligands displayed no impact on the growth of the fungal strain C. albicans during experimentation.
The metal complexes showed noteworthy enhancement antibacterial activity and were more toxic than the parent organic ligands against both the Gram-positive and the Gram-negative bacterial types. The metal complexes showed zones of inhibition in the range of 8-23 mm. The highest activity showed by the [Co(SL1)2] complex exhibited good activity against the Gram –ve bacteria P. mirabilis with zone of inhibition measured of 23 mm. In addition, the compounds [FeSL2 .2H2O] and [CoSL2] showed moderate activity against the Gram –ve bacteria P. mirabilis with zones of inhibitions of 22 and 21 mm respectively. Moreover, the complex [CoSL2] showed good activity against the Gram –ve bacteria P. aeruginosa with zone of inhibition equal to 21 mm. On comparing with the reference antibacterial drug Amoxicillin used for treatment of bacterial infections, the compounds showed lower antibacterial activity. The antifungal tests of the metal complexes indicated resistance of the microorganisms to the metal complex chemicals.
The remarkable enhancement in the antibacterial activity of the metal complexes compared to the organic Schiff base ligands might be referred to the impact of the transition metal ions in the metal complex compounds. The higher activity of the coordination compounds than the parent ligands can be also interpreted of the basis of Overtones permeability concept and Tweedy’s chelation theory [35]. The presence of metal ion in the complexes increases the permeability as the complexes dissolves in lipids and pass more easily into the cell. Moreover, according to Tweedy’s chelation theory, the polarity of the metal ions will be reduced to a considerable extent on coordination because of the overlap of the partial participation of the metal ion’s +ve charge with the orbitals of donor atoms of the ligands [36-37]. This behavior generate delocalization of π-electrons over the entire coordination sphere and hence foster the liposolubility of the metal complexes. This enhancement in the lipophilicity of the metal complexes make the penetration of the complexes into the lipid layers of the microbial cells and hence give rise to adverse effects in the cell environment and enzymes of the cell and further restrict the proliferation of the microorganism [10, 38]. In addition, the metal complexes hinder the respiration process of the cell and hence prevent the protein synthesis, which restrict further the organism growth. In addition, the possibility of formation of hydrogen bonds between the azomethine linkage and the cell components will cause an adverse effect on the natural cell processes. [17].
This variation in activity can be explained based on the difference in the cell wall structure difference between microorganisms and hence impermeability of the microorganism cells or in the differences in the ribosomes of the cell constituents [17-18, 36].
The high stability constant of the Co(II) complexes explains their exhibited higher activity compared to other coordination compounds [39]. It can be stated that the presence of different metal ions, different scaffold moieties and different donating groups that present in the ligands are influential factors in the microbial potentials of the coordination compounds. A photograph for the antibacterial testis shown in figure (4).
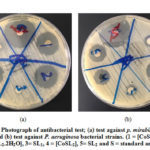 |
Figure 4: Photograph of antibacterial test; (a) test against p. mirabilis |
In Vivo Molluscicidal Assay
The ligands and their coordination compounds were examined in vivo for their molluscicidal potential versus the land snail named B. arabica. The results are presented in table (5). The Schiff base ligands showed activity at concentration of 1000 ppm while their metal complexes show activity at concentrations of 500 ppm. The complexes showed good activity at concentration of 1000 ppm and the highest activity was at concentration of 5000 ppm. It is observed that the coordination compounds were having higher toxic effect than their parent ligands, which perhaps interpreted explained because of the presence of metal ion that possibly have an influence the molluscicidal potential. The LC50 of the coordination compounds was higher at concentration of 1000 ppm and 5000 ppm compared to the free organic ligands.
Table 5: Molluscicidal potential of the selected ligands and their coordination compounds
|
Compounds |
No. of mortalities |
|||
|
100 ppm |
500 ppm |
1000 ppm |
5000 ppm |
|
|
SL1 |
— |
— |
1 |
2 |
|
SL2 |
— |
1 |
2 |
4 |
|
[CoSL1] |
— |
— |
— |
4 |
|
[NiSL1] |
— |
— |
2 |
4 |
|
[FeSL1.2H2O] |
— |
2 |
4 |
5 |
|
[CoSL2] |
— |
3 |
4 |
5 |
|
[NiSL2] |
— |
— |
4 |
4 |
|
[FeSL2.2H2O] |
— |
— |
3 |
5 |
Conclusion
In the present work, we prepared symmetrical tetradentate Schiff base ligands from 1,2-bis(2-aminphenythio)ethane condensed with 7-formy-8-hydroxyquinoline and 2-hydroxy-1-naphthaldehyde. The prepared Schiff base ligands were utilized in preparation of metal complexes with the iron triad transition metal ions (Fe(II), Co(II) and Ni(II)). The prepared compounds were characterized and their structures were proposed and confirmed using different physical and spectroscopic techniques. The analysis data suggested a square planar geometry for the Co(II) and Ni(II) metal complexes and octahedral geometry for Fe(III) complexes. The molar conductance study showed that metal complexes were non-electrolytic in nature. The antimicrobial observation data showed that the metal complexes were more biologically active compared to their parent organic Schiff base ligands. The complexes with cobalt metal ion showed highest activity against the Gram-negative bacterial strains. There was no activity against C. albicans fungal strains. The in vivo molluscicidal activity evaluation tests showed that both ligands and their corresponding metal complexes are having good molluscicidal activity against the tested snails. The metal complexes were more active than the parent free ligands.
Acknowledgments
The authors would like to thank the Chairman of the chemistry department for laboratory facilities and the Dean, Faculty of Science-Albaha University for encouragement.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Gupta, K.C.; Sutar, A.K. Coord Chem Rev. 2008, 252 (12-14), 1420–1450.
- Shafaatian, B.; Soleymanpour, A.; Kholghi O.N.; Notash, B.; Rezvani, S.A. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2014, 128, 363–369.
- Ejidike, I.P.; Ajibade, P.A. Rev Inorg Chem 2015, 35(4), 191–224.
- Sarkar, S.; Boceli, G.; Cantoni, A.; Ghosh, A. 2008, 27, 693–700.
- Khalaji, A.D.; Ghorbani, M.; Feizi, N.; Akbari, A.; Eigner, V.; Dusek M. Polyhedron. 2017, 121, 9-12.
- Alzahrani, A.A.; Zabin, S.A.; Jammali, M. Journal of Organic & Inorganic Chemistry. 2018, 4(1), 1-16.
- Khanmohammadi, H.; Rezaieam, K.; Amini, M.M.; Ng, S.W. Dyes and Pigments. 2013, 98, 557-564.
- Patel, V.K.; Vasanwala, A.M.; Jejurkar, C.R. Indian Journal of Chemistry. 1989, 28A(08), 719-721.
- Ismail, T. Coord. Chem. 2004, 58, 141-151.
- Zabin, S.A. Albaha University Journal of Basic and Applied Sciences. 2017, 1(2), 9-18.
- Abdelbaste, M.; Zabin, S.A.; Alorabi, A.Q. International Journal of Biology, Pharmacy and Allied Sciences. 2019, 8(3), 627-644.
- Alzahrani, A.A., Jammali, M.; El Mannoubi, I.; Zabin, S.A. International Journal of Biology, Pharmacy and Allied Sciences. 2019, 8(2), 319-341.
- He, P.; Wang, W.; Sanogo, B.; Zeng, X.; Sun, X.; Lv, Z.; Yuan, D.; Duan, L.; Wu, Z. Parasites & Vectors. 2017, 10:383, p2-11. DOI 10.1186/s13071-017-2313-3.
- El-Sherbini, G.T.; Zayed, R.A.; El-Sherbini, E.T. Parasitol. Res. 2009, vol. 2009, Article ID 474360, http://dx.doi.org/10.1155/2009/474360.
- Panchal, P.K.; Pansuriya, B.; Patel, M.N. Enzyme Inhib. Med. Chem. 2006, 21(4), 453-458.
- Wang, Y.; Yang, Z.; Wang, B. Met. Chem. 2005, 30, 879-883.
- Alaghaz, A.M.A.; Ammar, Y.A.; Bayoumi, H.A.; Aldhlmani, S.A. Mol. Struct. 2014, 1074, 359–375.
- Salehi, M.; Rahimifar, F.; Kubicki, M.; Asadi, A. Chim. Acta. 2016, 443, 28–35.
- More, G.; Raut, D.; Aruna, K.; Bootwala, S. Journal of Saudi Chemical Society. 2017, 21(8), 954–964.
- Ozkan, G.; Kose, M.; Zengin, H.; McKee, V.; Kurtoglu, M. Spectrochim Acta A Mol Biomol Spectrosc. 2015, 150, 966–973.
- Ejidike, I.P.; Ajibade, P.A. Chem. Appl. 2016, Vol. 2016, Article ID 9672451, 11 pages.
- Esmadi, F.T.; Khabour, O.F.; Abbas, K.; Mohammad, A.; Obeidat, R..T.; Mfady, D. Drug Chem Toxicol. 2016, 39(1), 41–47.
- Carabineiro, A.A.; Silva, L.C.; Gomes, P.T.; Pereira, L.J.; Veiros, L.F.; Pascu, S.I.; Duarte, M.T.; Namorado, S.; Henriques, R.T. Inorg Chem. 2007, 46(17), 6880-6890.
- Nartop, D.; Gurkan, P.; Sari, N.; Çete, S. Coord. Chem. 2008, 61(21), 3516-3524.
- Mahmoud, W.H.; Deghadi, R.G.; Mohamed, G.G. Res Chem Intermed. 2016, 42,7869-7907.
- El-Ansary, A.; Abdel-Fattah, H.M.; Abdel-Kader, N.S. Coord. Chem. 2008, 61(18), 2950–2960.
- Ansari, M.A.; Bhat, B.R. Chem. Sci. 2017, 129(9), 1483–1490.
- Liu, X.; Manzur, C.; Novoa, N.; Celedon, S.; Carrillo, D. Hamon, J-R. Coord Chem Rev. 2018, 357, 144–172.
- Erdem, E.; Sari, E.Y.; Kilincarslan, R., Kabay, N. Met Chem. 2009, 34, 167–174.
- Chaudary, N.K.; Mishra, P. Chem. Appl. 2017, Vol. 2017, Article ID 6927675, 13 pages.
- Hasan, M.R.; Hossain, M.A.; Salam, M.A.; Uddin, M.N. Journal of Taibah University for Science. 2016, 10, 766-773.
- Ayoub, M.A. Mol. Struct. 2018, 1173, 17-25.
- Andrew, F.P.; Ajibade, P.A. Mol. Struct. 2018, 1170, 24-29.
- Tajudeen, S.S.; Kannappan, G. Indian Journal of Advances in Chemical Science. 2016, 4(1), 40-48.
- Gupta, R.; Agrawal, N.; Gupta, K.C. Res J Pharm Biol Chem Sci. 2012, 3(2), 50-56.
- Al-Amiery, A.; Kadhum, A,A,H,; Mohamad, A. Chem. Appl. 2012, Volume 2012, Article ID 795812, 6 pages, doi:10.1155/2012/795812.
- Zabin, S.A.; Abdelbaset, M. J. Chem. 2016, 7(3), 322-328.
- Alias, M.; Kassum, H.; Shakir, C. Journal of the Association of Arab Universities for Basic and Applied Sciences. 2014, 15: 28–34.
- [El-Sherif, A.A.; Shoukry, M.M.; Abd-Elgawad, M.M.A. Spectrochim Acta A Mol Biomol Spectrosc.. 2012, 98, 307–321.

This work is licensed under a Creative Commons Attribution 4.0 International License.










