A Study of Antioxidant and Antimalarial Activity from the Bark of Voacanga Foetida BI. Rolfe, A Medicinal Plant from Lombok Island
Surya Hadi1* , Baiq Desy Ratnasari2
, Baiq Desy Ratnasari2 , Maulida Septiyana1
, Maulida Septiyana1 , Seto Priyambodo3
, Seto Priyambodo3 and I Made Sudarma1
and I Made Sudarma1
1University of Mataram, West Nusa Tenggara, Mataram, 83121, Indonesia
2Department of Pharmacy, Stikes Kusuma Bangsa Mataram, West Nusa Tenggara, Mataram, 83126, Indonesia
3Department of Medical Education, University of Mataram, West Nusa Tenggara, Mataram, 83121, Indonesia.
Corresponding Author E-mail: sur_hadi88@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/360324
Article Received on : 19 May 2020
Article Accepted on : 20 Jun 2020
Article Published : 25 Jun 2020
Voacanga foetida BI. Rolfe is one of the medicinal plant of Indonesia that are utilized as a traditional medicine. The traditional uses of V. foetida are a hint for an active metabolites presence in the plant that triggering the bioactive study. Hence, this research is designed to study the therapeutic activities of V. foetida and its metabolites that are responsible for those response. There were two bioactivities that was studied in this research, namely antioxidant and antimalarial. The bark of V. foetida was divided into three fractions, which were methanol extract, acid fraction, and base fraction obtained by acid-base extraction. The samples then tested using the DPPH method for the antioxidant activity and the Giemsa Staining method for microscopy against Plasmodium falciparum strain 3D7 for antimalarial activity. The result showed that the bark of V. foetida was weak in inhibiting the atomic radicals that concluding it as a low antioxidant. On the other hand, all three samples were showing a great activity in inhibiting P. falciparum 3D7 stain, especially the base fraction. GC-MS analysis identified that the base fraction and the methanol extracts contains an alkaloid lombine might responsible for the antimalarial activity. Future research will be directed to the antimalarial activity by exploring the responsible compounds in the base fraction.
KEYWORDS:Antioxidant; Antimalarial; Bark Extract; Voacanga Foetida
Download this article as:| Copy the following to cite this article: Hadi S, Ratnasari B. D, Septiyana M, Priyambodo S, Sudarma I. M. A Study of Antioxidant and Antimalarial Activity from the Bark of Voacanga Foetida BI. Rolfe, A Medicinal Plant from Lombok Island. Orient J Chem 2020;36(3). |
| Copy the following to cite this URL: Hadi S, Ratnasari B. D, Septiyana M, Priyambodo S, Sudarma I. M. A Study of Antioxidant and Antimalarial Activity from the Bark of Voacanga Foetida BI. Rolfe, A Medicinal Plant from Lombok Island. Orient J Chem 2020;36(3). Available from: https://bit.ly/2Vg9GKe |
Introduction
Voacanga foetida (BIume) Rolfe (Apocynacea) is a non-edible plant known as kumbi in few parts of Indonesia and distributed around Sumatera, Java, Borneo, and Lombok.1 Some people have been utilized the plant extract as traditional medicines to treat various skin diseases. The most utilized part of the plant are the leaves and the barks. These parts are usually mashed and placed in the infection spot to heal the wound, itches, and swelling.2 The traditional uses of V. foetida are a hint for an active metabolites presence in the plant that triggering the bioactive study. Hence, this research is designed to study the therapeutic activities of V. foetida and its metabolites that are responsible for those responses.
V. foetida is known for its alkaloid compounds and Hadi et al have identified various indole alkaloids in the bark of V. foetida such as voacangine, ibogamine, and a new alkaloid called lombine.3, 4 Alkaloid is one of the major compound group that possess various bioactivities and one of them is antimalarial. Quinine, is the first alkaloid that used as a malarial antidote commercially. It was isolated from the bark of Rubiaceae plant and cinchona tree. In addition, indole alkaloid from Apocynacea family has recorded to possess an anti-malarial activity such as ellipticine isolated from Aspidosperma vargasii barks. It was found to be highly active against Plasmodium falciparum and Plasmodium berghei with IC₅₀≤1.4 μM. 5 There is no data publication regarding antimalaria from V. foetida. Thus, this research was focusing to study the antimalarial activity in the bark extract of V. foetida.
Other secondary metabolites that were detected in the leaves and the barks extracts of V. foetida are steroid 6 and phenol.7, 8 Nevertheless, the research on V. foetida is more directed on alkaloid content. Thus, the phenol compound and its bioactivity in V. foetida has not been explored thoroughly. The phenolic compound has been known as antioxidant agent that quenching radical atom in the body. Thus, the second bioactivity that was studied in this research is the antioxidant activity of the V. foetida barks. To have a better understanding of the bioactivity of V. foetida barks, a metabolite screening was conducted on the basis of Gas Chromatography Mass Spectroscopy.
Material and Method
Sample Extraction
The sample was V. foetida barks collected from Tete Batu Village, East Lombok island of Indonesia. The dried sample of V. foetida was mashed into a fine powder, then extracted using methanol for 3×24 h. The crude methanol extract then re-extracted using the acid-base extraction method by adding CH3COOH 5%, DCM, and Na2CO3 10%, yielding base and acid fractions. The bioactivity of the methanol extract, the base fraction, and the acid fraction was tested for its antioxidant and anti-malaria activities. The antimalarial activity was assessed by the Giemsa Staining method for microscopy against Plasmodium falciparum strain 3D7 which are sensitive to chloroquine at Tropical Disease Diagnostic Center, Airlangga University. Meanwhile, the antioxidant activity of the bark extract was assessed using the DPPH method at Analysis Laboratory, Mataram University.
Antimalarial Assay
2 µl test solution with various concentration (1000 µg/ml, 100 µg/ml, 10 µg/ml, 1 µg/ml, 0.1 µg/ml, 0.01 µg/ml) were inserted into the well (well 96). 198 µl parasite were added into the well until the final concentration obtained are 100, 10, 1, 0.01, 0.001, 0.0001 µg/ml. The well then was put in the chamber and added with the mix gasses (O2 5%, CO2 5%, N2 90%). The chamber then incubated for 48 h at 37oC. The culture then harvested and analyzed using the Giemsa staining method.
Antioxidant Assay
The samples (methanol extract, acid fraction, and base fraction) were added with methanol 96%. The solution then incubated for 24 h in the dark place. Each solution then added with DPPH 0.1 Mm, incubated for 30 m in the room temperature at a dark place. The absorbance was measured at 517 nm. The procedure then repeated for the 20 h incubation.
Metabolite Screening
The metabolite presence in the bark of V. foetida was screened using Gas Chromatography Mass Spectrum (GCMS), Shimadzu QP2010 ULTRA, the RTX-5MS capillary column with a length of 30 m, diameter 0.25 mm and film thickness 0.25µm. Helium was used as the gas carrier with flow rate 30ml/min. The molecular mass range of ions was identified at 35-500 m/z. The temperatur programs: the injection temperature was set to 260oC with initial column started at 40oC for 5 minutes and programmed to increase 30oC per minute until it reached 260oC for 7 minutes.
Result and Discussion
Antimalarial Assay
The antimalarial test was conducted using the Giemsa Staining method for microscopy against Plasmodium falciparum strain 3D7 which are sensitive to chloroquine. The IC50 (µg/ml) data is displayed in Table 1.
Table 1: Antimalarial activity of V. foetida bark against P. falciparum 3D7 strain
|
No |
Sample |
Concentration (µg/ml) |
IC50 (µg/ml) |
||||||
|
100 |
10 |
1 |
0.1 |
0.01 |
0.001 |
0.0001 |
|||
|
1 |
Acid fraction |
100 |
100 |
65.01 |
65.01 |
30.84 |
15.56 |
6.28 |
0.13 |
|
2 |
Base fraction |
Lysis |
100 |
97.93 |
97.93 |
48.47 |
39.46 |
13.10 |
0.02 |
|
3 |
Methanol extract |
100 |
100 |
62.34 |
62.34 |
35.15 |
22.21 |
6.42 |
0.14 |
According to the WHO standard (2019), if IC50 of the antimalarial extract > 5 µg/ml the compound is classified as inactive, IC50 between 0.5 and 5 µg/ml the compound is classified as moderately active, IC50 < 0.5 µg/ml the compound is classified as an active compound. Based on Table 1, all the samples had IC50 <0.5 µg/ml which classify it as an active extract. The most active sample was a base fraction with IC50 0.02 µg/ml. The base fraction contains alkaloid compounds that probably responsible for the activity. In this extract, the alkaloid compound that was detected is new alkaloid compound called lombine (Table 4).
Hadi (2002) was found that all part of V. foetida was showing a negative result for antimalarial activity against P. falciparum K1.2 Thus, the extract might be active only against the 3D7 strain that was tested in this research. The 3D7 is originated from Africa, it is sensitive to the chloroquine. On the other hand, K1 strain is an Indian origin, a chloroquine-resistant. 8,9, 10 Thus, it can be concluded that V. foetida barks extracts, especially base fraction may be active against sensitive malaria strain and ineffective against drug-resistant strain.
The methanol extract and the acid fraction were showing a similar activity. As the initial extract, the methanol extract is supposed to contain all the metabolites that are fractionated in the acid and base fraction. Therefore, there is a huge possibility that the responsible compound for antimalarial activity in the methanol extract and other fraction are the same. However, as the methanol extract contains many compounds, there might be an antagonist compound that hindering the therapeutic activity. Hence, the antimalarial activity of methanol extract is lower compared to the base fraction. Thus, a further study regarding the synergist and antagonist activity among the compounds in methanol extract is interesting to conduct.
For the acid fraction, the antimalarial activity is mainly influenced by the fatty acid that is contained in the fraction. However, the possibility of other compounds that might be influencing the activity is worth to try. Hence, a further study is needed.
Antioxidant Assay
There were three samples of V. foetida bark that underwent an antioxidant activity test, which was base fraction, acid fraction, and crude methanol extract. The base fraction is containing indole alkaloids while the acid fraction is filled with fatty acids like palmitic acid, stearic acid, and many others as showed in the Table 4. The three samples that were incubated for 30 minutes were showing various antioxidant activity that is displayed in the table 2.
Table 2: Antioxidant assessment at 30-minute incubation
|
No. |
Sample |
Sample |
Absorbance |
% Inhibition |
Regression |
IC50 |
|
Concentration (ppm) |
( Y = bx+a) |
(ppm) |
||||
|
1 |
Control |
2.0 |
0.504 |
27.06 |
||
|
2 |
(Quercetin) |
4.0 |
0.405 |
41.39 |
Y= 7.54x + 11.62 |
|
|
3 |
6.0 |
0.301 |
56.44 |
R2= 0.999 |
8.18 |
|
|
4 |
8.0 |
0.185 |
73.23 |
|||
|
5 |
10.0 |
0.093 |
86.54 |
|||
|
6 |
Pa |
10.0 |
0.685 |
0.87 |
||
|
7 |
50.0 |
0.680 |
1.59 |
Y= 0.026x + 0.3848 |
||
|
8 |
100.0 |
0.672 |
2.75 |
R2= 0.9896 |
193.79 |
|
|
9 |
150.0 |
0.662 |
4.20 |
|||
|
10 |
200.0 |
0.651 |
5.79 |
|||
|
11 |
P1b |
10.0 |
0.329 |
37.92 |
||
|
12 |
50.0 |
0.309 |
41.70 |
Y= 0.0869x + 37.101 |
|
|
|
13 |
100.0 |
0.290 |
45.28 |
R2= 0.9969 |
148.40 |
|
|
14 |
150.0 |
0.262 |
50.57 |
|
||
|
15 |
200.0 |
0.242 |
54.34 |
|||
|
16 |
P2c |
10.0 |
0.315 |
36.62 |
|
|
|
17 |
50.0 |
0.285 |
42.66 |
Y= 0.0647x + 37.712 |
||
|
18 |
100.0 |
0.273 |
45.07 |
R2= 0.9309 |
189.92 |
|
|
19 |
150.0 |
0.263 |
47.08 |
|||
|
20 |
200.0 |
0.248 |
50.10 |
|
a Base fraction;b Acid fraction;c Methanol extract
IC50 0-50 ppm is means the extract has very strong activity, 50-100 ppm is strong; 100-150 ppm is moderate, and 150-200 ppm is weak. 12 According to that category, overall, V. foetida bark extract has low antioxidant activity. The IC50 value showed that the acid fraction (P1) had moderate activity (148.40 ppm) with the highest % inhibition of 52.34% at 200 ppm sample concentration. Meanwhile, the methanol extract (P2) was weak (189.92 ppm) with % inhibition around 50.10% at 200 ppm sample concentration. Furthermore, the base fraction (P) has the weakest activity with IC50 193.79 ppm and 5.79% at 200 ppm sample concentration. Compared to the standard (Quercetin), the three fractions of the bark have less antioxidant activity than that, indicating weak activity belong to the bark.
To look at more promising the antioxidant activity, the acid fraction and the methanol extract that had moderate to weak activity were re-tested using the same method with addition inhibition time to 20 h and the result was shown in Table 3. The variable of incubation time was showing different data in both samples. The acid fraction (P1) had increasing antioxidant activity (IC50 148.40 ppm to 84.26 ppm) from moderate to strong antioxidant. The methanol extract was showing a decreasing activity at 20 h incubation (189.92 ppm to 320.62 ppm), from weak antioxidant to very weak. The methanol extract might be had a degradation of chemical composition during the incubation. The acid fraction (P1) is more stable than that of methanol extract (P2) and need more time to act as antioxidant. Overall, the antioxidant activity of V. foetida bark is by far lower than that of the quercetin, the positive control (8.18 ppm).
Table 3: Antioxidant assessment result of base and acid fractions at 20 h Incubation
|
No. |
Sample |
Sample concentration |
Absorbance |
% Inhibition |
Regression |
IC50 |
|
(ppm) |
( Y = bx+a) |
(ppm) |
||||
|
1 |
Control |
2.0 |
0.401 |
27.06 |
||
|
2 |
(Quercetin) |
4.0 |
0.395 |
41.39 |
Y= 7.54x + 11.62 |
|
|
3 |
6.0 |
0.371 |
56.44 |
R2= 0.999 |
8,18 |
|
|
4 |
8.0 |
0.35 |
73.23 |
|||
|
5 |
10.0 |
0.292 |
86.54 |
|||
|
6 |
P1b |
10.0 |
0.365 |
38.24 |
|
|
|
7 |
50.0 |
0.326 |
44.84 |
Y= 0.01576x + 36.721 |
||
|
8 |
100.0 |
0.279 |
52.79 |
R2= 0.9968 |
84.26 |
|
|
9 |
150.0 |
0.241 |
59.22 |
|||
|
10 |
200.0 |
0.184 |
68.87 |
|
||
|
11 |
P2c |
10.0 |
0.361 |
33.76 |
Y= 0.01576x + 33.071 |
|
|
12 |
50.0 |
0.351 |
35.6 |
R2= 0.9904 |
320.62 |
|
|
13 |
100.0 |
0.335 |
38.53 |
|||
|
14 |
150.0 |
0.325 |
40.37 |
|
||
|
15 |
200.0 |
0.305 |
44.04 |
|
a Base fraction;b Acid fraction; c Methanol extract
Metabolite Screening
The three extracts of the bark of V. foetida was analyzed to identify their metabolites using GC-MS and the chemical composition is displayed in the Table 4.
Table 4: Metabolites that were detected in V. foetida barks
|
No |
Extract |
% Area |
SI (%) |
|
Methanol extract |
|||
|
1 |
Hexadecanoic acid |
28.35 |
95 |
|
2 |
Unknown compound, identified as lombine |
7.07 |
No hit compound* |
|
3 |
9-octadecenal, (Z) |
31.17 |
90 |
|
4 |
Octadecanoic acid |
11.45 |
86 |
|
5 |
1,2-Benzenedicarboxylic acid, bis (2-ethyl |
21.96 |
96 |
|
Acid fraction |
|||
|
1 |
1,1-bibicyclo(2,2,2)octyl-4-carboxylic acid |
4.18 |
92 |
|
2 |
2-chlorocyclohexanol |
1.35 |
90 |
|
3 |
Hexadecanoic acid, methyl ester |
3.83 |
94 |
|
4 |
Octadec-9-enoic acid |
4.26 |
89 |
|
5 |
Hexadecanoic Acid |
26.34 |
95 |
|
6 |
8,11-octadecadienoic acid, methyl ester |
5.00 |
90 |
|
7 |
9,12-octadecadienoic acid |
31.67 |
91 |
|
8 |
Octadecanoic acid |
8.22 |
93 |
|
9 |
1,2-Benzenedicarboxylic acid |
8.37 |
96 |
|
Base fraction |
|||
|
1 |
Stigmast-5-en-3-ol, (3 beta, 24S) |
36.45 |
82 |
|
2 |
Stigmast-5-en-3-ol, (3 beta, 24S) |
38.52 |
87 |
|
3 |
Unknown compound, identified as Lombine |
25.02 |
No hit compound* |
*identified on the basis of comparation of ion fragmentation pattern to reff 2
Generally, the extracts are consisting of fatty acid, plant sterol, acid compounds, alcohol, and the unknown compound identifies as lombine. The fatty acid was the responsible compound for the antioxidant activity. Thus, acid fraction has the strongest activity among other extracts. However, the fatty acid bioactivity is not as strong as the phenolic compound in attracting the radical atom. The absence of phenolic compound in the extract was the reason for their low antioxidant activity.
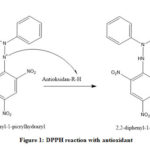 |
Figure 1: DPPH reaction with antioxidant |
Phenolic compound is a stronger antioxidant than that of fatty acid because of its electron density around the aromatic part that create a resonance in which make it easier to donate the electron to the radical atom.11
 |
Figure 2: Electronegativity of phenol |
As for the antimalarial activity, the most active sample was a base fraction with IC50 0.02 µg/ml. The base fraction contains alkaloid compounds that probably responsible for the activity. As it is known, alkaloid quinine was the first antimalarial drug from a natural product that is actively used till now.12 Based on the GCMS, the unknown compound on the base fraction has been identified on the basis of comparation to ion fragmentation pattern to reference Hadi and Bremner (2006).
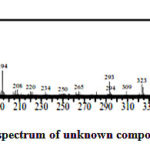 |
Figure 3: Fragmentation spectrum of unknown compound of the base fraction |
According to Hadi and Bremner (2006) and Hadi et al (2018), those pattern of ion fragmentation is the characteristic of alkaloid lombine. It is proved by the presence of lombine skeleton ion fragments, namely m/z 172 or/and 208. Furthermore, there is fragmentation of m/z 352 as a characteristic of alkaloid lombine molecular weight. In addition, the spectrum of m/z 130 and 144 are the characteristic of unsubstituted alkaloid indoles moiety, the fragmentation of m/z 337 and 293 are indicating the presence of methyl and carbomethoxy group respectively. Moreover, it gives high peak at m/z 44 and 180.
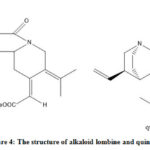 |
Figure 4: The structure of alkaloid lombine and quinine |
Thus, the antimalarial activity of the base fraction is predicted to be influenced by the alkaloid lombine. According to the Lipinski rule, lombine is following the rule of five that support the druglikeness of the compound (Table 5). A QSAR analysis is needed to study more about the pharmacology of lombine as antimalarial.
Table 5: Lipinski Rule of Lombine and Quinine
|
Variable |
Standard (Lipinski Rule) |
Quinine |
Lombine |
|
Mass (Dalton) |
<500 |
324.18 |
312 |
|
Hydrogen bond acceptors |
<10 |
3 |
6 |
|
Hydrogen bond donors |
<5 |
1 |
5 |
|
Log P |
<5 |
2.6 |
-0.053101 |
Conclusion
V. foetida bark is more suitable to be developed as an antimalarial agent than that of antioxidants. The three extracts of V. foetida bark were highly active inhibiting the P. falciparum strain 3D7. The antimalarial activity is mostly influenced by the alkaloid compound identified as lombine found in the methanol extract and base fraction. Thus, future research will be more focused to explore the use of the bark as sources of antimalarial agents.
Acknowledgement
We would like to give sincere gratitude to MENRISTEK (Ministry of Research and Technology) of Indonesia for funding this research.
Conflict of Interest
The authors declare that this research has no conflict of interest.
References
- http://portal.cybertaxonomy.org Voacanga foetida. http://portal.cybertaxonomy.org/flora-malesiana/cdm_dataportal/taxon/4748601d-1005-4fb7-a134-b98c964d83f1/specimens (accessed 14 Mei 2020)
- Hadi, S. University of Wollongong Thesis Collection 1954-2016.2002
- Hadi, S.; Bremner, J.B. Natural Product Communications. 2006, 1 (10):825-829
- Julianto, D. UNRAM Press. 2018
- e Silva, L.R.; Montoia, A.; Amorim, R.C.N.; Melo, M.R.; Henrique, M.C.; Nunomura, S.M.; Costa, M.R.F.; Neto, V.A.; Costa, D.S.; Dantas, G. and Lavrado, J. Phytomedicine, 2012.20(1), pp.71-76
- Adriani, S.E.; Sari, R.K. Jurusan Farmasi Uhamka, 2012
- Hadi, S.; Kurniawati, L.; Mariana, B.; Muliasari, H.; Rahayu, S. Proceedings of The 9th Joint Conference on Chemistry, Universitas Diponogoro, UNDIP Press: Universitas Diponogoro. 2015
- Ditya, V.Y.; Hadi, S.; Murniati. Universitas Mataram, UNRAM Press. 2019
- Molina-Cruz, A.; DeJong, R.J.; Ortega, C.; Haile, A.; Abban, E.; Rodrigues, J.; Jaramillo-Gutierrez, G.; Barillas-Mury, C. Proceedings of the National Academy of Sciences. 2012, 109 (28), E1957-E1962
- Agarwal, P.; Anvikar, A.R.; Pillai, C.R.; Srivastava, K. The Indian journal of medical research. 2017, 146 (5), 622
- Kajiyama, T.; Ohkatsu, Y. Polymer degradation and stability.2001, 71 (3), 445-452
- Pinheiro, L.; Feitosa, L.M.; Silveira, F.F.; Boechat, N. Anais da Academia Brasileira de Ciências. 2018, 90 (1), 1251-1271

This work is licensed under a Creative Commons Attribution 4.0 International License.









