A Facile and Efficient Tamarind Juice Catalyzed One- Pot Synthesis of Benzopyranopyrimidines in Aqueous Medium Just by Grinding: A Green Chemistry Approach
Y. I. Shaikh1* , S. S. Shaikh
, S. S. Shaikh 2, Khursheed Ahmed1
2, Khursheed Ahmed1 , G. M. Nazeruddin1
, G. M. Nazeruddin1 and V. S. Shaikh3
and V. S. Shaikh3
1Department of Chemistry and P.G. Research Centre, Abeda Inamdar Sr. College, Pune
2Department of Chemistry, Gramonnati Mandal’s Arts, Comm. and Sci. College, Narayangaon.
3Department of Chemistry, Nowrosjee Wadia College, Pune.
Corresponding Author E-mail: sheray2k@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/360308
Article Received on : 29 Mar 2020
Article Accepted on : 10 Jun 2020
Article Published : 11 Jun 2020
A comprehensive and efficient Tamarind (Tamarindous indica) juice catalysed one pot synthesis of benzopyranopyrimidines in aqueous medium through condensation of 4-Hydroxy-coumarin (10mmol), various substituted benzaldehydes (10mmol)) and urea/thiourea (20mmol) just by grinding at ambient condition is reported in excellent yield of the product. This is a green chemistry approach, which is need of the hour.
KEYWORDS:Benzopyranopyrimidine; Multi-Component Reaction; Grinding Technique (Green Chemistry); Tamarind Juice
Download this article as:| Copy the following to cite this article: Shaikh Y. I, Shaikh S. S, Ahmed K, Nazeruddin G. M, Shaikh V. S. A Facile and Efficient Tamarind Juice Catalyzed One- Pot Synthesis of Benzopyranopyrimidines in Aqueous Medium Just by Grinding: A Green Chemistry Approach. Orient J Chem 2020;36(3). |
| Copy the following to cite this URL: Shaikh Y. I, Shaikh S. S, Ahmed K, Nazeruddin G. M, Shaikh V. S. A Facile and Efficient Tamarind Juice Catalyzed One- Pot Synthesis of Benzopyranopyrimidines in Aqueous Medium Just by Grinding: A Green Chemistry Approach. Orient J Chem 2020;36(3). Available from: https://bit.ly/30y0rZu |
Introduction
The industrial revolution1 took place between 1760 to 1840 and transition of hand power to machine took place and all the chemical reactions were accomplished by using steam power means coal was used and due to materialistic approach of various industries nobody was bothered about pollution problem and as a result of this ill effects were observed on health of human beings and pollution of this planet was increasing day by day. All the scientists of the world came forward and new term was coined as Green Chemistry2.So under the umbrella of green chemistry to accomplish any chemical reaction an organic/medicinal chemist has to follow green chemistry principles. It means they have to prefer multicomponent reaction3 than multi step reaction so that the atom economy of the reaction could be nicely controlled. Similarly a biocatalyst4 and water5 as a solvent is preferable. A reaction always should consume less energy i.e. supported by grinding technique,6 micro-wave7 or ultrasound irradiation8 with excellent yield of the product.
Pyrimidine derivatives have attracted interest of an organic/medicinal chemist because of their various biologically active properties.9, 10, 11 Patil etal12, 13 reported the synthesis of pyrimidine using lemon juice and pineapple juice as a catalyst. Whereas Nazeruddin14, 15etal reported the same reaction catalyzed by Tamarind juice under ultra sound and Grape juice just by grinding at ambient conditions.
Coumarin derivatives possess a variety of biologically active properties,16 and if these two rings i.e. pyrimidine and coumarin are condensed the resulting heterocyclic compound’s derivatives would posses’ enhanced biological activities. Various protocols are reported in the literature17 .However, most of them do not follow the basic principles of green chemistry. In the present work rather it is continuation of our earlier work18 there is condensation of tautomeric form of 4-Hydroxycoumarin 1, aromatic aldehyde 2 and urea/ thiourea 3 in aqueous medium catalyzed by Tamarind juice just by grinding technique furnishes benzopyrimidines 4 in excellent yields (Scheme 1)
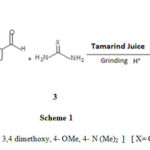 |
Scheme 1 |
Experimental Section
The chemicals required to carry out this research work were purchased from Merck and Loba and used as it is. .Melting points were determined by an open capillary method and are uncorrected.IR spectra were recorded on Perkin–Elmer FT-IR-1710 instrument.1H NMR spectra were recorded using CDCl3or DMSOd6 as a solvent and TMSas an internal standard either on BrukerAC-200 MHz or Bruker MSL-300 MHz instrument. . Elemental analyses were determined by an elemental
Preparation of Aqueous Extract of Tamarind Juice
The tamarind fruit’s pulp (without cover and seeds) was purchased from the local market and out of it 5 g of the pulp was soaked in 50 ml water for half an hour followed by pealing with hand to take out the extract and centrifuged by (REMI RM-12C). The clear portion of the aqueous extract (pH=3) was used as catalyst.
General Procedure for the Preparation of Benzopyranopyrimidines:
The aromatic aldehyde (2mmol) and 4-hydroxycoumarin (2mmol) and urea/thiourea (4mmol) were taken in mortar followed by addition of 10 ml 10% tamarind juice the mixture was ground for appropriate time (Table-1). The completion of the reaction was monitored by thin layer chromatography. The crude product was filtered, washed by water and dried under vacuum followed by crystallization using ethanol as a solvent.
Results and Discussion
An environmentally benign procedure for synthesis of benzopyranopyridines is developed by condensing, 4-hydroxycoumarin, aromatic aldehydes and urea/thiourea in aqueous medium just by grinding using tamarind juice as a catalyst (scheme-I).There is a complex role of tamarind juice in promoting the coupling reaction. Moreover, acidic nature of the juice (H+ ion) accelerates the reaction. The mechanism of the reaction is suggested in scheme-II. Further, the methodology is general because, it accommodates various substituted benzaldehydes with electron donating and electron withdrawing groups (Table-1).Furthermore, the products are obtained in excellent yields just by filtration followed by crystallization.
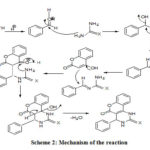 |
Scheme 2: Mechanism of the reaction |
The reaction is initiated by protonation of the aldehyde followed by a formation of condensed product with urea/thiourea, which reacts with 4-hydroxy coumarin to accomplish the final product, the benzopyranopyridine
Table 1: Data of benzopyranopyrimidines (4a-4h) accomplished by condensing, 4-hydroxycoumarin (1), aromatic aldehydes-(2a-2h) and urea/thiourea (3) in aqueous medium just by grinding using tamarind juice as a catalyst
|
Entry of the product4a -4h |
R |
X |
Time (min) |
Yield (%) |
Obs.M.P.0C |
Lit.M.P. 0C |
|
4a |
phenyl |
O |
30 |
92 |
160-162 |
162-16419 |
|
4b |
2-chloro phenyl |
O |
20 |
85 |
205-207 |
205-20718 |
|
4c |
4-chloro phenyl |
O |
20 |
95 |
198-200 |
197-19820 |
|
4d |
3,4-dimethoxy phenyl |
O |
40 |
90 |
268-270 |
270-27219 |
|
4e |
2-hydro phenyl |
S |
40 |
91 |
169-171 |
169-17118 |
|
4f |
4-methoxy phenyl |
S |
35 |
90 |
264-266 |
264-26618 |
|
4g |
4-N,N dimethyl phenyl |
O |
45 |
90 |
239-241 |
240-24220 |
|
4h |
phenyl |
S |
30 |
91 |
188-190 |
188-18919 |
structures of the products were confirmed by comparing their M.P. /B.P. and spectral data with authentic samples, which are as follows.
Spectral and Elemental Analysis Data
3, 4-Dihydro-4-Phenyl-1H-Chromeno [4, 3-d] Pyrimidine-2, 5-Dione (4a). White solid, m.p. 160-1620C. IR: (KBr) (νmax cm-1)=3410, 2924, 2727, 2360, 1654, 1459, 1379, 1303, 1154, 1075, 964, 722 cm-1; 1H-NMR (DMSO): δ(ppm)=6.34(s, 1H, CH), 7.16-7.59(m, 9H, Ar-H), 7.87(brs, 1H, NH), 7.9(brs, 1H, NH); Anal. Calcd for C17H12N2O3: C, 69.86; H, 4.14; N, 9.58%. Found: C, 69.92; H, 4.22; N, 9.70%.
4-(2-Chlorophenyl)-3, 4-Dihydro-1H-Chromeno [4, 3-d] Pyrimidine-2, 5-Dione (4b). White solid, m.p. 205-2070C. IR: (KBr) (νmax cm-1)= 3402, 3300, 3040, 1682, 1607, 1159, 1219, 1060, 757, 652, 553, 493, 453 cm-1; 1H-NMR (DMSO): δ(ppm)= 6.14(s,1H,CH), 7.1-7.53(m, 8H, Ar-H), 7.84(brs,1H,NH), 7.86(brs,1H, NH);Anal. Calcd for C17H11ClN2O3:62.49; H, 3.39; N, 8.57%. Found: C, 62.58; H, 3.51; N, 8.65%.
4-(4-Chlorophenyl)-3, 4-Dihydro-1H-Chromeno [4, 3-d] Pyrimidine-2, 5-Dione (4c). White solid, m.p. 198-2000C. IR: (KBr) (νmax cm-1)= 3400, 3079, 2893, 2839, 2733, 2615, 1668, 1608, 1566, 1491, 1450, 1350, 1309, 1217, 1093, 1055, 1014, 765, 671 cm-1; 1H-NMR (DMSO): δ(ppm)= 6.45(s,1H, CH),7.28-7.66(m,8H,Ar-H),7.94(brs,1H,NH), 7.98(brs,1H,NH); Anal. Calcd for C17H11ClN2O3: C, 62.49; H, 3.39; N, 8.57%. Found: C, 62.55; H, 3.47; N, 8.66%.
3,4-Dihydro-4-(3,4-Dimethoxyphenyl)-1H-Chromeno[4,3-d]Pyrimidine-2,5-Dione (4d). White solid, m.p. 268-2700C. IR: (KBr) (νmax cm-1)= 3415, 2938, 2835, 2728, 2611, 2363, 1699, 1617, 1506, 1453, 1346, 1244, 1187, 1126, 1010, 907, 763, 506, 452 cm-1;1H-NMR(DMSO):δ(ppm)= 3.54(s,3H,OCH3),3.69(s,3H,OCH3),6.25(s,1H,CH),6.64-7.86(m,7H, Ar-H), 7.88 (brs,1H,NH), 7.89(brs,1H,NH); Anal. Calcd for C19H16N2O5: C,64.77; H, 4.58; N, 7.95%. Found: C, 64.73, H, 4.69; N, 7.99%.
1, 2, 3, 4-Tetrahydro-4-(2-Hydroxyphenyl)-2-Thioxochromeno [4, 3-d] Pyrimidin-5-One (4-e).Pale yellow, m.p. 169-171 0C. IR: (KBr) (νmax cm-1)=3411, 3071, 2362, 1752, 1606, 1488, 1449, 1389, 1343, 1241, 1271, 1039, 940, 865, 752, 465 cm-1; 1H-NMR (DMSO): δ(ppm)= 3.3(brs,1H,OH), 6.89-7.85(m,9H,Ar-H), 8.32(brs,1H,NH), 10.67(brs,1H,NH); Anal. Calcd forC17H12N2O3S: C, 62.95; H, 3.73; N, 8.64; S, 9.89%. Found: C, 63.07; H, 3.84; N, 8.73; S, 9.91%.
1, 2, 3, 4-Tetrahydro-4-(4-Methoxyphenyl)-2- Thioxochromeno [4, 3-d] Pyrimidin-5-One (4-f). White solid, m.p. 264-2660C. IR: (KBr) (νmax cm-1)=3401, 3072, 1967, 1714, 1626, 1608, 1583, 1489, 1450, 1330, 1344, 1344, 1242, 1217, 1174, 1039, 941, 866, 808, 754, 678, 624, 464 cm-1; 1H-NMR (DMSO): δ(ppm)= 3.78(s, 3H,OCH3), 6.43(s,1H,CH), 6.86-7.71(m,8H,Ar-H), 8.02(brs, 1H, NH), 8.06(brs,1H, NH); Anal. Calcd for C18H14N2O3S: C, 63.89; H, 4.17; N, 8.28; S, 9.48%. Found:C, 63.96; H, 4.26, N, 8.38; S, 9.53%.
4-(4-(Dimethylamino) Phenyl)-3, 4-Dihydro-1H-Chromeno [4, 3-d] Pyrimidine-2, 5-Dione (4-g). Pinkish brown, m.p.239-2410C. IR: (KBr) (νmax cm-1)= 3426, 3088, 2885, 2727, 2609, 1662, 1610, 1568, 1523, 1450, 1348, 1307, 1207, 1184, 1089, 1053, 958, 906, 765, 744 cm-1; 1H-NMR (DMSO): δ(ppm)= 3.19(s,6H,CH3),6.44(s,1H,CH), 7.24-7.37(m,8H,Ar-H), 7.86(brs,1H, NH),7.90(brs,1H,NH); Anal. Calcd forC19H17N3O3: C, 68.05; H, 5.11; N, 12.53%.Found: C, 68.13; H, 5.18; N, 12.60%.
1, 2, 3, 4-Tetrahydro-4-Phenyl-2-Thioxochromeno [4, 3-d] Pyrimidin-5-One (4-h). White solid, m.p.188-1900C. IR: (KBr) (νmax cm-1)= 3450, 2923, 2854, 1656, 1463, 1377, 1303, 1155, 970, 727 cm-1; 1H-NMR (DMSO): δ(ppm)= 6.36(s,1H,CH), 7.17-7.60 (m, 9H,Ar-H), 7.88(brs,1H,NH), 7.91 (brs, 1H, NH); Anal. Calcd for C17H12N2O2S: C, 66.22; H, 3.92; N, 9.08;S, 10.40 %. Found: C, 66.31; H, 3.95; N, 9.13; S, 10.44%.
Conclusion
A facile, efficient, green chemistry protocol for the synthesis of benzopyranopyrimidines is developed with simple procedure, low cost, high yield of the product and above all such protocols are the need of the hour.
Acknowledgement
Authors are thankful Maharashtra Cosmopolitan Education Society Pune for the financial support.
Conflict of Interest
The authors have declared that there is no conflict of interest.
References
- Anthony WrigleyE;Journal of Interdisciplinary History, 2018,01,09-42.
- Anastas, P.T.; Warner,J.C.;Oxford University Press, Oxford, 1998.
- Shaikh, S.R.;Nazeruddin, G.M.;Chemical and Pharmaceutical Research,2014,6(12),505-534.
- Andrade, L. H.; Utsunomiya, S.; Omori, A. T.; Porto, A. L. M.; Comasseto, J. V. J.; Catal. B: Enzyme,2006,38, 84-90.
- Sherman, J.; Chin, B.; Huibers, P. D. T.; Garcia-Valls, R.; Hatton, T. A.; 1998,106, 253-271.
- Bose, A.K.;Pednekar, S.;Ganguly, S.N., Chakraborty, G.;Manhas, M.S.;Tetrahedron Lett., 2004,45,8351–8353.
- Nazeruddin, G. M.;Mulani, S. S.;Pandharpatte, M. S.;Chinese journal of Chemical Society,2012,59,645-649.
- Al-Kadasi, A. M. A.;Nazeruddin, G.M;Asian Journal of Chemistry, 2011, 23(7), 3155-3157.
- Motos, L.H.S.; Masson, F.T.; Simeoni,L.A.;Homem-de-Mello,M.; Eur J Med Chem.2018,143,1779-1789.
- Kappe, C.O.; Tetrahedron,1993,49(32), 6937–6963.
- Kappe, C. O.; Chem. Res., 2000,33, 879–888.
- Patil,S.; Jadhav, S.D.; and Deshmukh, M.B.; Archive of Applied Science,2011,3,203-208.
- Patil, S.; Jadha, S.D.; and Mane, S.Y.; J. of Org. Chemistry,2011,1,125-131.
- Nazeruddin,G.M.;Shaikh,Y.I.;Der Pharmacia Sinica, 2014,5(6),64-68.
- Nazeruddin,G.M.; Mulani,S.S.; Shaikh, Y.I.; Shaikh, S.S.K.; J of Scientific Research in Science and Technology;2017, S.I.3,46-50.
- Gupta, A.S.;Prabhu, B.S.;Phull, M.S.;Indian J. Chem. Sect. B., 1996, 35, 170-171.
- Matache, M.;Dobrota,C.;Bogdan, N. D.;Dumitru, I.;Ruta, L.L.; Paraschivescu, C. C.;Farcasanu, I. C.;Baciu,I.;Funeriu, D. P.;Tetrahedron,2009, 65, 5949-5957.
- Al-Kadasi,A.; and Nazeruddin,G. M.; of Chem. Pharma. Res.,2013,5(7),204-210.
- Brahmbhatt, D.I.; Roliji, G.B.; Pandya, S.U.; Pandya, U.R.; Indian J.Chem., 1999, 38, 839.
- Kidwai, M.;Sapra,P.;Synthetic communications.,2002, 32, 1639-1645.

This work is licensed under a Creative Commons Attribution 4.0 International License.









