Design, Spectral and Antibacterial Investigations of Some Mixed Ligand Metal Complexes
Debapriya Pradhan1, Susanta K. Biswal1*and Ranjan K. Mohapatra2
1Centurion University of Technology and Management, Odisha, India
2Department of Chemistry, Government College of Engineering, Keonjhar, Odisha, India
Corresponding Author E-mail: dr.skbiswal@cutm.ac.in
DOI : http://dx.doi.org/10.13005/ojc/360218
Article Received on : 23 Feb 2020
Article Accepted on : 26 Mar 2020
Article Published : 02 May 2020
A novel series of mixed ligand Co(II), Cu(II), and Zn(II) complexes of the type [M(L1)(L2) Cl].2H2O, where HL1= 2-(o-vanillinidenehydrazino)benzimidazole (primary ligand) and L2 = 2,2′-bipyridine (secondary ligand) have been synthesized and investigated by various physico-analytical techniques. The spectral results suggested tridentate nature of the Schiff base and coordinated to the metal ions via azomethine nitrogen, ring nitrogen and deprotonated phenolic oxygen atom. These complexes possess distorted octahedral coordination geometry. Furthermore, antibacterial activities for these compounds against B. subtilis, E. coli and S. typhi have been evaluated. In addition, QSAR and electrostatic potential surface analysis of the Schiff base is carried out.
KEYWORDS:Antifungal and Theoretical Studies; Mixed Ligand Complex; Schiff Base; Spectral
Download this article as:| Copy the following to cite this article: Pradhan D, Biswal S. K, Mohapatra R. K. Design, Spectral and Antibacterial Investigations of Some Mixed Ligand Metal Complexes. Orient J Chem 2020;36(2). |
| Copy the following to cite this URL: Pradhan D, Biswal S. K, Mohapatra R. K. Design, Spectral and Antibacterial Investigations of Some Mixed Ligand Metal Complexes. Orient J Chem 2020;36(2). Available from: https://bit.ly/35zEs4V |
Introduction
Benzimidazole is a bicyclic compound containing two nitrogen atoms at alternate positions fused to the benzene ring. It is used as an important precursor for the synthesis of various biologically active heterocyclic compounds. Its derivatives have been found to display remarkable antiviral [1], antimicrobial [2], antioxidant[3], anticancer [4] and antiulcer [5] activities. The effectiveness of such compounds increases when coordinated to metal ions [6-8]. Now-a-days, mixed ligand metal complexes have received considerable attention for their wide range of applications in analytical and biological chemistry [9-12].
Furthermore, the mixed ligand metal complexes of 2,2’-bipyridine have also been reported [13-15]. Considering the versatile nature of such compounds and our ongoing work on them [16-19], we have made an effort to report the synthesis of some ternary complexes having benzimidazole moiety. Moreover, these ternary compounds exhibited moderate antibacterial activity when screened against some selected strains; B. subtilis, E. coli and S. typhi.
Materials and Methods
The chemicals and reagents were obtained from Sigma Aldrich and used as such.
Preparation of Ligand
The precursor 2-hydrazinobenzimidazole was prepared as reported previously [8]. The Schiff base ligand; 2-(o-vanillinidenehydrazino)benzimidazole was prepared by condensing 2-hydrazinobenzimidazole with o-vanillin [8].
Yield 80%, color yellow; Anal. Calc. (%): C, 63.83; H, 4.96; N, 19.86, Found (%): C, 63.76; H, 4.92; N, 19.82.
Preparation of Mixed Ligand Complexes
Hot ethanolic solution of the Schiff base (0.01 mol, 20 mL), metal(II) chloride (0.01 mol, 20 mL) and 2,2’-bipyridine (0.01 mol, 20 mL) in 1:1:1 molar ratio are mixed with stirring. The resulting mixture was refluxed for 2 hours at pH 7-8 by adding 0.25 g of solid NaOH. It was filtered, washed and finally, dried in vacuo. The schematic representation for the synthesis of mixed ligand complexes is shown in Fig. 1.
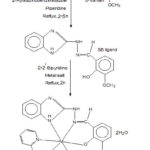 |
Figure 1: Scheme for the preparation of metal complexes |
Analysis and Physical Measurements
The UV and FTIR spectra were obtained from Perkin-Elmer and Varian FTIR spectrophotometer, respectively. The CHN analysis was done in MLW-CHN micro analyzer. The 1H-NMR spectra in DMSO-d6 were recorded on JEOL GSX-400 equipment. Thermal analysis was carried out by Netzch-429 thermal analyser and ESR spectra were recorded on E-112 EPR Spectrophotometer.
Results and Discussion
These compounds were stable, non hygroscopic and soluble in DMSO and DMF. The physico-analytical data for them is shown in Table 1.
Table 1: Physico-analytical data of the compounds
|
Compounds |
Yield (%) |
Λam |
C Found (Calcd) |
H Found (Calcd) |
N Found (Calcd) |
M Found (Calcd) |
|
[CoL1L2] 2H2O |
67 |
10.2 |
52.82 (52.86) |
4.37 (4.40) |
14.75 (14.80) |
10.33 (10.39) |
|
[CuL1L2] 2H2O |
65 |
11.4 |
52.41 (52.45) |
4.35 (4.37) |
14.64 (14.68) |
11.05 (11.10) |
|
[ZnL1L2] 2H2O |
61 |
7.5 |
52.28 (52.31) |
4.31 (4.36) |
14.78 (14.65) |
11.27 (11.33) |
aOhm-1 cm2 mole-1
IR Spectra
The coordination of phenolic oxygen is confirmed from the disappearance of band at ~3300 cm-1 due to phenolic -OH vibration for metal complexes. The band for vN-H (exocyclic) remains unchanged in the metal complexes, indicates non-involvement of imino nitrogen in coordination. Further, the non coordination of ring nitrogen atom (-C=N) of benzimidazole moiety in the complexes is also ascertained as there is no change in the positions of bands at ~ 1540 and ~ 1320 cm-1 was observed. On the other hand, the band at ~3150 cm-1 for benzimidazole νN-H group shifted to lower frequency region by ~20 cm-1, indicated the involvement of -NH group in coordination. Besides the above, the coordination of azomethine nitrogen is also identified due to shifting of bands for vC=N (azomethine) and vN-N vibrations. Further, an additional band was appeared at ~2840 cm-1 for –OCH3 group in all the compounds, which remain unaltered. The absorption band for vO-H (lattice water) is assigned at ~3500 cm-1. In addition, a sharp band at 670–685 cm-1 for ν(C═N) of pyridine is also observed for ternary complexes.[20] Some importantIR data for the ternary compounds are listed in Table 2. The representative IR spectrum of [ZnL1L2]2H2O is shown in Fig. 2.
Table 2: Important IR data for the ternary compounds
|
[CoL1L2] 2H2O |
[CuL1L2] 2H2O |
[ZnL1L2] 2H2O |
Assignments |
|
3500 |
3510 |
3510 |
νO-H (lattice water) |
|
~3140 |
~3140 |
~3130 |
νN-H (benzimidazole) |
|
~ 1525 |
~ 1530 |
~ 1520 |
νC=N (benzimidazole) |
|
~ 1320 |
~ 1335 |
~ 1340 |
vC-N (cyclic) |
|
1610 |
1615 |
1610 |
νC=N (azomethine) |
|
655 |
660 |
668 |
νC=N (pyridine) |
|
1270 |
1270 |
1282 |
νC-O (phenolic) |
|
580 |
570 |
585 |
νM-N |
|
490 |
495 |
510 |
νM-O |
|
348 |
345 |
330 |
νM-Cl |
![Figure 2: IR spectrum of [ZnL1L2] 2H2O](http://www.orientjchem.org/wp-content/uploads/2020/05/Vol36No2_Des_Deb_Fig2-150x150.jpg) |
Figure 2: IR spectrum of [ZnL1L2] 2H2O |
Thermal Analysis
The TGA data for the ternary complexes are listed in Table 3. The compounds displayed similar type of thermal decomposition and remains unaffected up to ~50 followed by a slight depression up to ~110 . This confirms the presence of lattice water in the metal complexes. The anhydrous metal complexes showed rapid degradation after 270 for organic constituents and continues up to ~600 as indicated in the plateau of the thermo gram. Finally reaches to the corresponding stable metal oxide.
Table 3: TGA data for the ternary compounds
|
Compounds |
Temp. range of Water loss () |
% of water loss |
Decomposition Temperature () |
% of residue |
Composition of the residue |
||
|
Found |
Calc. |
Found |
Calc. |
||||
|
[CoL1L2] 2H2O |
50-110 |
6.28 |
6.34 |
260 |
13.17 |
13.21 |
CoO |
|
[CuL1L2] 2H2O |
60-110 |
6.25 |
6.29 |
270 |
13.85 |
13.90 |
CuO |
|
[ZnL1L2] 2H2O |
55-100 |
6.24 |
6.27 |
245 |
14.08 |
14.12 |
ZnO |
Electronic Spectra
The mixed ligand Co(II) complex displayed two main bands around ~10,800 cm-1 (broad) and ~22,500 cm-1 (strong) under octahedral symmetry. The magnetic moment value for the Co(II) complex is obtained at 4.8 B.M. The Cu(II) complex in DMSO displayed two bands at ~14,600 cm-1 and ~16,750 cm-1. In this case, the band for 2B1g®2A1g is not observed. The magnetic moment value for Cu(II) complex is obtained at 1.85 B.M. The data supports hexa coordinated environment for the compounds. The electronic data for the ternary complexes are listed in Table 4.
Table 4: Electronic data for the ternary compounds
|
Compounds |
ν (cm-1) |
Transition |
Geometry |
μeff (BM) |
|
[CoL1L2] 2H2O |
~10,800 (broad) |
4T1g(F)®4T2g(F) (v1) |
Octahedral |
4.8 |
|
~22,500 (strong) |
4T1g(F)® 4T1g (P) (v3) |
|||
|
[CuL1L2] 2H2O |
~14,600 |
2B1g®2B2g (v2) |
Distorted octahedral |
1.85 |
|
~16,750 |
2B1g®2Eg (v3) |
1H NMR Spectra
The 1H NMR spectra of HL1 (Fig. 3) and Zn(II) complex displayed a multiplet at δ 7.4-8.5 ppm for aromatic protons of phenyl groups. The signals at δ 6.7 ppm and δ 9.0 ppm are due to ring NH and exocyclic NH protons, respectively. Also the signals for -OCH3 and azomethine protons appeared at δ 4.5 ppm and δ 8.7 ppm, respectively. The downfield shift of ring -NH and azomethine protons indicates their participation in coordination. Furthermore, the disappearance of the signal due to phenolic -OH proton in the metal complexes confirms its participation in co-ordination through deprotonation.
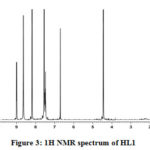 |
Figure 3: 1H NMR spectrum of HL1 |
Electron Spin Resonance (ESR) Spectra
The ESR spectrum of Cu(II) complex at 300 K displayed a well resolved four line spectrum for the compound. Also the data supported mononuclear nature of the compound. The spin Hamiltonian parameters were determined, which showed g‖ = 2.25 > g┴ = 2.07 > 2.0023. The data suggests octahedral geometry for the chelate [21]. Also, g‖ < 2.3 suggests covalent nature of metal-ligand bond and the unpaired electron is present in dx2-y2 orbital [22]. In addition, the exchange interaction term G (3.66) was determined.
In Vitro Antibacterial Activity
The in vitro antibacterial activities of these studied compounds were reported at 100 μg mL-1 concentrations by Agar Well method [23] against B. subtilis, E. coli and S. typhi. The zone of inhibition is shown in Table 5 and Fig. 4. The standard antibacterial drug ciprofloxacin was also screened as above. The Schiff base ligand showed moderate activity against all organisms, whereas the ternary complexes displayed greater activity. This will be explained by chelation theory and C=N bonds. Moreover, other factors such as solubility, dipole moment, nature of the ligand and geometry may contribute towards the higher activity [24, 25].
Table 5: Antibacterial activity of the compounds
|
Compounds |
B. subtilis |
E. coli |
S. typhi |
|
HL1 |
13.54 |
13.26 |
14.92 |
|
[CoL1L2] 2H2O |
17.25 |
19.38 |
22.34 |
|
[CuL1L2] 2H2O |
21.48 |
20.62 |
24.52 |
|
[ZnL1L2] 2H2O |
14.75 |
17.56 |
19.15 |
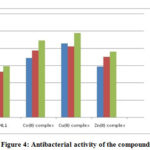 |
Figure 4: Antibacterial activity of the compounds |
Electrostatic Potential
The ESP surface of HL1 ligand was analyzed by using ArgusLab 4.0.1 software and shown in Fig. 5. The surface contains number of possible sites for electrophilic attack. The study displayed very specific data about the charge distribution. The negative regions are mainly over -OCH3 group oxygen, phenolic oxygen, azomethine nitrogen and benzimidazole nitrogen atoms as shown by red colour. The -OCH3 group oxygen atom is not involved in coordination due to steric effect, which was confirmed earlier.
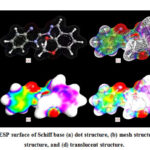 |
Figure 5: The ESP surface of Schiff base |
Conclusion
The foregoing observations suggest that all the mixed ligand complexes are neutral in nature and contain lattice water molecules. The spectral study revealed octahedral geometry for all the metal complexes in which the primary ligand behaves as tridentate chelation with the metal ion. The metal complexes showed moderate antibacterial activity compared to the Schiff base ligand. The higher activity is due to the enhanced lipophilic property of the central metal ion for chelation. Moreover, the electrostatic potential surface of the Schiff base is analyzed, which also confirms tridentate chelation of the ligand.
Acknowledgement
The authors are highly thankful to the authorities of Centurion University of Technology and Management, Odisha, India for providing necessary facilities with financial assistance.
Conflicts of Interest
There are no conflicts to declare.
References
- Tonelli, M.; Paglietti, G.; Boido, V.; Sparatore, F.; Marongiu, F.; Marongiu, E.; La Colla, P.; Loddo, R. Biodivers., 2008, 5, 2386-2401.
- Ozkay, Y.; Tunai, Y.; Karaka, H.; Isikdag, I. J. Med. Chem., 2010, 45, 3293-3298.
- Odame, F.; Krause, J.; Hosten, E. C.; Betz, R.; Lobb, K.; Tshentu, Z. R.; Frost, C. L. Chem. Soc. Ethiop., 2018, 32, 271-284.
- Salahuddin; Shaharyar, M.; Mazumder, A.; Ahsan, M. J. Arabian J. Chem., 2014, 7, 418-424.
- Cho, S. Y.; Kang, S. K.; Kim, S. S.; Cheon, H. G.; Choi, J. K.; Yum, E. K. Korean Chem. Soc., 2001, 22, 1217-1223.
- Wu, H.; Yuan, J.; Bal, Y.; Pan, G.; Wang, H.; Shao, J.; Gao, J.; Wang, Y. Coord. Chem., 2012, 65, 4327-4341.
- Mohapatra, R. K.; Dash, M.; Mishra, U. K.; Mahapatra, A.; Dash, D. C. React. Inorg. Metal-Org. Nano-Met. Chem., 2014, 44, 642-648.
- Mohapatra, R. K.; Das, P. K.; El-ajaily, M. M.; Mishra, U.; Dash, D. C. Chem. Soc. Ethiop., 2018, 32(3), 437-450.
- El-ajaily, M. M.; Maihub, A. A.; Mahanta, U. K.; Badhei, G.; Mohapatra, R. K.; Das, P. K. Rasayan J. Chem., 2018, 11(1), 166-174.
- Sigel, H. Chem. Int. Ed., 1975, 14, 394–402.
- Rao, T. P.; Reddy, L. P.; Pillai, A. R. Talanta, 1998, 46, 765-813.
- Fathima, S. S. A.; Meeran, M. M. S.; Nagarajan, E. R. Molecular Liq., 2019, 279, 177–189.
- Garoufis, A.; Koutsodimou, A.; Katsaros, N.; Mitsopoulou, C.-A.; Hadjiliadis, N. Polyhedron, 1999, 18, 361-369.
- Omar, M. M.; El-Halim, H. F. A.; Khalil, E. A. M. Organometal. Chem., 2017, 31, e3724.
- Eremina, J. A.; Lider, E. V.; Samsonenko, D. G.; Sheludyakova, L. A.; Berezin, A. S.; Klyushova, L. S.; Ostrovskii, V. A.; Trifonov, R. E. Chim. Acta., 2019, 487, 138–144.
- Azam, A.; Al-Resayes, S. I.; Wabaidur, S. M.; Trzesowska-Kruszynska, A.; Kruszynski, R.; Mohapatra, R. K.; Siddiqui, M. R. H. Chim. Acta., 2018, 471, 698–704.
- Mohapatra, R. K.; Ghosh, S.; Naik, P.; Mishra, S. K.; Mahapatra, A.; Dash, D. C. Korean Chem. Soc., 2012, 56, 62-67.
- Mohapatra, R. K.; Sarangi, A. K.; Azam, M.; El-ajaily, M. M.; Zahan, M. K.; Patjoshi, S. B.; Dash, D. C. Mol. Struct., 2019, 1179, 65–75.
- El-ajaily, M. M.; Sarangi, A. K.; Mohapatra, R. K.; Hassan, S. S.; El-daghare, R. N.; Mohapatra, P. K.; Raval, M. K.; Das, D.; Mahal, A.; Cipurkovic, A. Al-Noor, T. H. ChemistrySelect., 2019, 4, 9999–10005.
- Abd El-halim, H. F.; Omar, M. M.; Mohamed, G. G. Acta. Part A, 2011, 78, 36-44.
- Nagakavitha, D.; Reddy, K. H. Indian Chem. Soc., 2015, 92, 71-78.
- Raman, N.; Kulandaisamy, A.; Jayesubramanian, K. Indian J. Chem., 2002, 41A, 942-949.
- Rahman, A., Choudry, M. I.; Thomsen, W. J. Bioassay Techniques for Drug Development, Harwood Academic Publishers, Netherlands, 2001.
- El-Gamel, N. E. A. Coord. Chem., 2010, 63, 534-543.
- Shakdofa, M. M. E.; Al-Hakimi, A. N.; Elsaied, F. A.; Alasbahi, S. O. M.; Alkwlini, A. M. A. Chem. Soc. Ethiop., 2017, 31, 75-91.

This work is licensed under a Creative Commons Attribution 4.0 International License.









