A Facile Synthesis of Pyrrolidine-Based Iminosugars as Potential Alpha-Glucosidase Inhibitors
Muhamad Zulfaqar Bacho1, Mohd Fazli Mohammat2, Zurina Shaameri2, Agustono Wibowo3, Firdaus Kamarulzaman4 and Ahmad Sazali Hamzah2*
1Faculty of Applied Sciences, Universiti Teknologi Mara (UiTM), 40450 Shah Alam, Selangor Darul Ehsan, Malaysia.
2Organic Synthesis Laboratory, Institute of Science, Universiti Teknologi MARA (UiTM), 42300 Bandar Puncak Alam, Selangor Darul Ehsan, Malaysia.
3Faculty of Applied Sciences, Cawangan Pahang,Universiti Teknologi Mara (UiTM), 26400 Bandar Jengka, Pahang, Malaysia.
4Natural Products Division, Forest Research Institute Malaysia (FRIM), Kepong, 52109 Selangor Darul Ehsan, Malaysia.
Corresponding Author E-mail: asazali@uitm.edu.my
DOI : http://dx.doi.org/10.13005/ojc/360214
Article Received on : 25 Mar 2020
Article Accepted on :
Article Published : 30 Apr 2020
A multifaceted approach comprising MCR (multicomponent reaction), amination and stereoselective reduction reactions was used to synthesize new pyrrolidine-based iminosugars. The key step of this strategy involves the contruction of a highly functionalised pyrroldine ring skeleton through MCR approach. Subsequently, amination and reduction reactions to the ring skeleton provide a quick access to new pyrrolidine- based imino sugars. The iminosugars were then tested against alpha glucosidase activity in which one compound (4-((4-methoxyphenyl)amino)pyrrolidin-3-ol), was found to be the most potent at low dosage.
KEYWORDS:Alpha-Glucosidase; Antidiabetic; Iminosugars; Pyrrolidine
Download this article as:| Copy the following to cite this article: Bacho M. Z, Mohammat M, F, Shaameri Z, Wibowo A, Kamarulzaman F, Hamzah A. S. A Facile Synthesis of Pyrrolidine-Based Iminosugars as Potential Alpha-Glucosidase Inhibitors. Orient J Chem 2020;36(2). |
| Copy the following to cite this URL: Bacho M. Z, Mohammat M, F, Shaameri Z, Wibowo A, Kamarulzaman F, Hamzah A. S. A Facile Synthesis of Pyrrolidine-Based Iminosugars as Potential Alpha-Glucosidase Inhibitors. Orient J Chem 2020;36(2). Available from: https://bit.ly/3d3oLVM |
Introduction
Diabetes mellitus (DM) is a continuously rising chronic metabolic disorder and has become a vital problem to the world population1. Over time, this disease has escalated the number of health issues among patients commonly to the type 2 diabetes (T2D)2. T2D happens by an abnormal postprandial increase of blood glucose level due to failed insulin production or action3. One way to overcome this calamity is by using alpha-glucosidase inhibitors, an approach to prevent postprandial hyperglycemia since they can act as competitive inhibitors of small intestinal brush-border alpha-glucosidases4,5. This glucosidase can inhibits the enzymes and delay the conversion of polysaccharide into absorbable monosaccharides (glucose and fructose)6. Some alpha-glucosidases such as acarbose, voglibose and miglitol that are currently available in the market prove the effectiveness of this approach (Figure1)7. Due to the limited number of these commercially available inhibitors, new alternative synthetic methods are thus required to combat this disease effectively.
For the past decade, many groups have reported the synthesis of pyrrolidine-based iminosugars8. Zhang and co-workers reported the synthesis of iminosugars using D-glucose as it’s main precursor. The target molecule (3S,4S)-3-((R)-1-2-dihydroxyethyl)pyrrolidine-3,4-diol was obtained in 10 steps with 24 % yield9. Doddi and co-workers reported the synthesis of azasugars utilizing pyrrolidine skeleton followed by regiospecific amination, ring closing metathesis, and diastereospecific dihydroxylations as the key reactions. These sugar molecules however, were found to have moderate inhibition against glycosidase enzyme6. In addition other synthetic strategies of pyrrolidine-based iminosugars have also been reported10,11,12.
In continuation of our work on the five membered heterocycle system7,8 ,13, we now report the synthesis of pyrrolidine based iminosugars in short steps via multi component reaction (MCR), amination, stereoselective reduction.
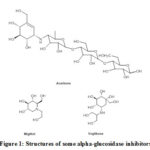 |
Figure 1: Structures of some alpha-glucosidase inhibitors |
Experimental
General
Infrared spectra (IR) was recorded on NICOLET 6700 FT-IR using diamond with ATR. NMR spectra was recorded on JEOL Resonance ECZ400 [400 MHz (1H) and 100 MHz (13C)] using TMS as the internal standard. The molecular weight of synthesized compound was recorded on Agilent Technnologies model: 6560 Accurate-Mass Q-TOF LC/MS, Thermo Scientific Orbitrap Fusion Tribid Mass Spectrometer and GC-MS Agilent Technologies 7693 Autosampler (GC System). Elemental analysis was performed by Flash 2000 Organic Elemental Analyzer. Melting point was run by Stuart SMP30. Analytical TLC was performed on silica gel 60 F254, Merck (layer thickness 0.25 mm, Merck) and visualized with UV light and KMnO4 as the detecting agent.
General Procedure for the Synthesis of Pyrrolidine-Based Iminosugar Intermediates (4a-k).
A mixture of compound 1 (1 equiv) and aldehyde (1 equiv) together with an equimolar amount of amine in ethanol was refluxed towards completion (0.5 – 2 h). Iced-water was added to the mixture after cooling and HCl was then added dropwise to pH 1. Filter the solid while appear. Traces aldehyde in the crude product was washed with water and ether to give (4a-k).
Ethyl 4-Hydroxy-5-Oxo-2, 5-Dihydro-1H-Pyrrole-3-Carboxylate (4a)
White solid; 60%; m.p. 106-109○C. IR (ATR) n/cm-1: 3344 (OH), 2986 (NH, amide), 1782 (C=O, ester), 1687 (C=C), 1670 (-N-C=O, amide), 1302 (C-N); 1H-NMR (400 MHz, CDCl3): δ 4.91-4.85 (2H, s, CH2), 4.39-4.32 (2H, q, J= 7.2 Hz, CH2), 1.38-1.31 (3H, t, J= 7.1 Hz, CH3); 13C-NMR (100 MHz, CDCl3,): δ 166.54 (COH), 164.23 (C=O) 151.29 (C=O), 116.09 (quat. C), 66.26 (OCH2), 62.09 (CH2), 14.34 (CH3); Anal. Calcd. for C7H9NO4: C, 49.12; H, 5.30; N, 8.18; O, 37.39. Found: C, 49.30; H, 4.65; N, 7.04; O, 39.01; GCMS m/z (EI, + ve): found 172.00 ([M]+), C7H9NO4 calculated 172.06.
Ethyl 4-Hydroxy-1-Methyl-5-Oxo-2,5-Dihydro-1H-Pyrrole-3-Carboxylate (4b)
Yellowish solid; 40%; m.p. 139-141○C. IR (ATR) n/cm-1: 3400 (-OH), 1780 (C=O, ester), 1648 (C=C), 1274 (C-N), 734; 1H-NMR (400 MHz, CDCl3): δ 1.33 (3H, t, J= 7.2 Hz, CH3), 3.07 (3H, s, NCH3), 3.96 (2H, s, CH2), 4.31 (2H, q, J= 7.2 Hz, OCH2); 13C-NMR (100 MHz, CDCl3): δ 14.30 (CH3), 30.10 (NCH3), 48.10 (CH2), 61.20 (OCH2), 107.60 (quat. C), 157.40 (C=O), 164.10 (C=O), 165.20 (COH); Anal.Calcd. for C8H11NO4 (185.07): C, 51.89; H, 5.99; N, 7.56; O, 34.56. Found: C, 51.80; H, 4.65; N, 7.70; O, 35.85.
Ethyl 2-Ethyl-4-Hydroxy-5-Oxo-2,5-Dihydro-1H-Pyrrole-3-Carboxylate (4c)
White solid; 12.93%; m.p. 146-148○C. IR (ATR) n/cm-1: 3305 (OH), 3176 (NH, amide), 1766 (C=O, ester), 1678 (C=C), 1639 (N-C=O, amide), 1454 (CH2), 1371 (CH3), 1304 (C-N); 1H NMR (400 MHz, CDCl3): δ 4.31-4.25 (m, 1H), 4.28-4.17 (m, 2H), 2.05-1.90 (m, 1H), 1.66 (td, J = 14.4, 7.3 Hz, 1H), 1.28 (tdd, J = 14.9, 7.2, 1.0 Hz, 3H), 0.85-0.69 (m, 3H); 13C NMR (100 MHz, CDCl3): δ 167.31 (COH), 164.05 (C=O), 155.28 (C=O), 112.47 (quat. C), 60.24 (OCH2), 54.37 (CH), 24.79 (CH2), 13.32 (CH3), 7.04 (CH3); Anal. Calcd. for C9H13NO4: C, 54.26; H, 6.58; N, 7.03; O, 32.13. Found: C, 53.69; H, 6.49; N, 6.91; O, 32.91; GCMS m/z (EI, + ve): found 199.00([M]+), C9H13NO4 calculated 199.08.
Ethyl 4-Hydroxy-2-Isopropyl-5-Oxo-2,5-Dihydro-1H-Pyrrole-3-Carboxylate (4d)
White solid; 29.57%; m.p. 165-167○C. IR (ATR) n/cm-1: 3318 (OH), 3177 (NH, amide), 2925 (C-H), 1768 (C=O, ester), 1722 (C=C), 1630 (N-C=O, amide), 1372 (CH3); 1H-NMR (400 MHz, CDCl3): δ 4.31 – 4.22 (m, 2H), 4.19 (d, J = 2.8 Hz, 1H), 2.42 (dtd, J = 13.9, 7.0, 2.8 Hz, 1H), 1.30 (s, 3H), 1.07 (d, J = 7.1 Hz, 3H), 0.59 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 167.85 (COH), 164.13 (C=O), 155.31 (C=O), 112.83 (quat. C), 60.28 (OCH2), 58.64 (CH), 29.01 (CH), 19.83 (CH3), 13.18 (CH3); Anal. Calcd. for C10H15NO4: C, 56.33; H, 7.09; N, 6.57; O, 30.01. Found: C, 55.30; H, 6.89; N, 6.17; O, 31.64; GCMS m/z (EI, + ve): found 213.10 ([M]+), C10H15NO4 calculated 213.10.
Ethyl 4-Hydroxy-5-Oxo-2-(p-Tolyl)-2,5-Dihydro-1H-Pyrrole-3-Carboxylate (4e)
Light yellow solid; 28.04%; m.p. 153-156○C. IR (ATR) n/cm-1: 3303 (OH), 2982 (NH, amide), 1719 (C=O, ester), 1621 (C=C) 1556 (N-C=O, amide), 667 (Ar-CH3); 1H-NMR (400 MHz, CDCl3): δ 7.12 (s, 2H), 7.07 (s, 2H), 4.34 (q, J = 7.2 Hz, 2H), 4.14 (dd, J = 7.1, 1.6 Hz, 1H), 2.31 (d, J = 9.6 Hz, 3H), 1.36 (t, J = 7.1 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 170.59 (COH), 165.52 (C=O), 160.36 (C=O), 140.56 (aromatic. C), 137.29 (aromatic. C), 129.45 (CH-Ar), 127.14 (CH-Ar), 107.97 (quat. C), 64.11 (OCH2), 56.85 (CH), 21.25 (CH-Ar), 13.89 (CH3); Anal. Calcd. for C14H15NO4; C, 61.85; H, 5.88; N, 4.81; O, 27.46. Found: C, 55.10; H, 5.12; N, 3.37, O, 36.41; GCMS m/z (EI, + ve): found 284.1 ([M + Na]+), C14H15NO4 calculated 284.09.
Ethyl 2-(4-Cyanophenyl)-4-Hydroxy-5-Oxo-2,5-Dihydro-1H-Pyrrole-3-Carboxylate (4f)
Yellow solid; 20.49%; m.p. 139-141○C. IR (ATR) n/cm-1: 3302 (OH), 2987 (NH, amide), 1728 (C=O, ester), 1591 (C=C), 1501 (N-C=O), 768 (Ar-CN); 1H-NMR (400 MHz, CD3OD): δ 7.76-7.61 (2H), 7.54-7.39 (2H), 5.37-5.22 (1H), 4.12-3.99 (2H), 1.17-1.00 (3H); 13C NMR (100 MHz, CD3OD): δ 167.39 (COH), 165.66 (C=O), 163.10 (C=O), 143.63 (quat. C), 132.14 (CH-Ar), 128.29 (CH-Ar), 118.43 (CN), 111.66 (quat. C), 110.38 (quat. C), 60.67 (CH2), 39.46 (CH), 12.94 (CH3); Anal. Calcd. for C14H12N2O4; C, 61.76; H, 4.44; N, 10.29; O, 23.51. Found: C, 58.36; H, 4.31; N, 6.92; O, 30.41: GCMS m/z (EI, + ve): found 274.2 ([M + 2H]+), C14H12N2O4 calculated 274.09.
Ethyl 4-Hydroxy-2-(4-Methoxyphenyl)-1-Methyl-5-Oxo-2,5-Dihydro-1H-Pyrrole-3-Carboxylate (4g)
Light Yellow; 58%; m.p. 154-156○C. IR (ATR) n/cm-1: 3105 (OH), 2927 (NH, amide), 1673.84 (C=O, ester), 1612 (C=C), 1512 (N-C=O), 776 (Ar-OMe); 1H-NMR (400 MHz, CDCl3): δ 7.07 (d, 2H), 6.86 (d, J = 6.9 Hz, 2H), 4.95 (s, 1H), 4.13 (q, J = 7.2 Hz, 2H), 3.80 (s, 3H), 2.79 (s, 3H), 1.13 (t, J = 7.1 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 165.40 (COH), 159.95 (C=O), 157.87 (C=O), 128.80 (CH-Ar), 126.47 (aromatic. C), 114.25 (CH-Ar), 112.12 (quat. C), 62.14 (OCH2), 61.06 (CH), 55.39 (OCH3), 27.65 (CH3-N), 14.04 (CH3); Anal. Calcd. for C15H17NO5; C, 61.85; H, 5.88; N, 4.81; O, 27.46. Found: C, 61.40; H, 5.81; N, 4.00; O, 28.69; GCMS m/z (EI, + ve): found 293.10 ([M + 2H]+), C15H17NO5 calculated 293.13.
Ethyl 4-Hydroxy-2-(4-Methoxyphenyl)-5-Oxo-2,5-Dihydro-1H-Pyrrole-3-Carboxylate (4h)
Light yellow solid; 25.71%; m.p. 152-153○C. IR (ATR) n/cm-1: 3302 (OH), 3004 (NH, amide), 1685.55 (C=O, ester), 1612 (C=C), 1514.43 (N-C=O), 759 (Ar-OMe); 1H-NMR (400 MHz, CDCl3): δ 7.15 (dd, J = 6.6, 2.1 Hz, 2H), 6.84 (dd, J = 6.6, 2.1 Hz, 2H), 5.20 (d, J = 0.9 Hz, 1H), 4.15 (q, J = 7.0 Hz, 2H), 3.78 (s, 3H), 1.15 (t, J = 7.1 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 170.70 (COH), 160.28 (C=O), 159.67 (C=O), 137.20 (aromatic. C) 135.93 (aromatic. C), 129.05 (CH-Ar), 114.32 (CH-Ar), 108.06 (quat. C), 64.17 (OCH2), 55.39 (OCH3), 39.98 (CH), 13.89 (CH3); Anal. Calcd. for C14H15NO5; C, 60.64; H, 5.45; N, 5.05; O, 28.85. Found: C, 61.40; H, 5.81; N, 4.00; O, 28.79; GCMS m/z (EI, + ve): found 278.10 ([M + H]+), C14H15NO5 calculated 278.10.
Ethyl 4-Hydroxy-1-(2-Hydroxyethyl)-5-Oxo-2,5-Dihydro-1H-Pyrrole-3-Carboxylate (4i)
Light orange; 34.60%; m.p. 132-134○C. IR (ATR) n/cm-1: 3479 (-CH2OH), 3310 (-CH-OH), 2920 (NH, amide) , 1694 (C=O, ester), 1655 (C=C), 1513 (N-C=O); 1H-NMR (400 MHz, CD3OD): δ 4.26 (q, J = 7.2 Hz, 2H), 4.13 (s, 2H), 3.76-3.68 (m, 2H), 3.62-3.51 (m, 2H), 1.29 (t, J = 7.1 Hz, 3H); 13C NMR (100 MHz, CD3OD): δ 176.98 (COH), 165.88 (C=O), 163.69 (C=O), 107.90 (quat. C), 60.32 (OCH2), 59.42 (CH2OH), 48.30 (CH2-N), 45.32 (CH2), 13.27 (CH3); Anal.Calcd. for C9H13NO5; C, 50.23; H, 6.09; N, 6.51; O, 37.17. Found: C, 48.98; H, 5.91; N, 5.87; O, 39.24; GCMS m/z (EI, + ve): found 215.00 ([M]+), C9H13NO5 calculated 215.08.
Ethyl 1-Butyl-4-Hydroxy-5-Oxo-2,5-Dihydro-1H-Pyrrole-3-Carboxylate (4j)
White Solid; 25%; m.p. 109-111○C. IR (ATR) n/cm-1: 3099 (OH), 2956 (NH, amide), 1663 (C=O, ester), 1447 (-CH2-), 1362 (-CH3), ; 1H-NMR (400 MHz, CDCl3): δ 4.30 (q, J = 7.2 Hz, 2H), 3.97 (t, J = 15.8 Hz, 2H), 3.48 (t, J = 7.5 Hz, 2H), 1.57 (s, 2H), 1.32 (t, J = 7.3 Hz, 5H), 0.95-0.88 (m, 3H); 13C NMR (100 MHz, CDCl3): δ 165.09 (COH), 164.24 (C=O), 156.80 (C=O), 107.68 (quat. C), 61.15(OCH2), 46.24 (CH2-N), 42.89 (CH2), 30.35 (CH2), 19.99 (CH2), 14.35 (CH3), 13.75 (CH3); Anal. Calcd. for C11H17NO4; C, 58.14; H, 7.54; N, 6.16; O, 28.16. Found: C, 53.85; H, 7.04; N, 5.06; O, 34.05; GCMS m/z (EI, + ve): found 227.10 ([M]+), C11H17NO4 calculated 227.12.
Ethyl 4-Hydroxy-1-(4-Hydroxyphenyl)-5-Oxo-2,5-Dihydro-1H-Pyrrole-3-Carboxylate (4k)
Brownish yellow solid; 5%; m.p. >160○C decomposed. IR (ATR) n/cm-1: 3235 (OH), 2990 (NH, amide), 1654 (C=O, ester), 1595 (C=C), 1514 (N-C=O), 757 (Ar-OH); 1H-NMR (400 MHz, CD3OD): δ 7.49 (d, J = 8.7 Hz, 2H), 6.80 (d, J = 9.1 Hz, 2H), 4.40 (s, 2H), 4.28 (d, J = 6.9 Hz, 2H), 1.31 (t, J = 7.1 Hz, 3H); 13C NMR (100 MHz, CD3OD): δ 169.17 (COH), 166.68 (C=O), 161.33 (C=O), 155.31 (quat. C), 130.39 (quat. C), 121.87 (CH-Ar), 115.28 (CH-Ar), 110.34 (quat. C), 60.37 (OCH2) 47.07 (CH2), 13.32 (CH3); Anal.Calcd. for C13H13NO5; C, 59.31; H, 4.98; N, 5.32; O, 30.39. Found: C, 51.64; H, 4.92; N, 4.07; O, 29.37; GCMS m/z (EI, + ve): found 286.90 ([M + Na]+), C13H13NO5 calculated 286.07.
General Procedure for the Synthesis of Pyrrolidine-Based Iminosugar Intermediates (5a-c).
Mixtures of 0.01 mole of 2,3-dioxopyrrolidine (4a), 0.012 mole of amine, 0.016 mole of formic acid were heated at reflux for 16 hours. Each solution was concentrated by distillation to approximately 10 ml and diluted with water while still hot until faint turbidity appeared. The products which crystallized from the mixture upon cooling, were collected by filtration and purified by recrystallization in ethanol-water mixture.
Ethyl 5-Oxo-4-(Phenylamino)-2,5-Dihydro-1H-Pyrrole-3-Carboxylate (5a)
Yellow solid; 21.55%; m.p. 59-62○C. IR (ATR) n/cm-1: 3396 (NH), 1700 (C=O, ester), 1675 (C=C), 1539 (N-C=O), 758 (NH-Ar); 1H NMR (400 MHz, CDCl3): δ 7.30 (t, 2H), 7.14 (t, J = 7.5 Hz, 1H), 7.08 (d, J = 7.3 Hz, 2H), 4.93 (s, 2H), 4.23 (q, J = 7.2 Hz, 2H), 1.25 (t, J = 7.1 Hz, 3H); 13C NMR (100 MHz, CDCl3) 167.51 (C=O), 163.89 (C=O), 137.85 (quat. C), 137.61 (aromatic. C), 128.72 (CH-Ar), 125.10 (CH-Ar), 122.73 (CH-Ar), 111.83, (quat. C), 60.94 (OCH2), 48.46 (CH2), 14.28 (CH3); GCMS m/z (EI, + ve): found 247.10 ([M + H]+), C13H14N2O3 calculated 247.11.
Ethyl 4-((4-Ethylphenyl)Amino)-5-Oxo-2,5-Dihydro-1H-Pyrrole-3-Carboxylate (5b)
Reddish brown oily; 36.06%; IR (ATR) n/cm-1: 3328 (NH), 1766 (C=O, ester), 1685 (C=C), 1517 (N-C=O), 1459 (CH2), 1356 (CH3), 759 (NH-Ar-CH2CH3); 1H-NMR (400 MHz, CDCl3): δ 7.13 (d, J = 8.5 Hz, 2H), 7.01 (d, J = 6.2 Hz, 2H), 4.93 (s, 2H), 4.23 (q, J = 7.2 Hz, 2H), 2.62 (q, J = 7.6 Hz, 2H), 1.28-1.19 (m, 6H); 13C NMR (100 MHz, CDCl3) δ 141.35 (C=O), 137.92 (C=O), 135.36 (quat. C), 128.12 (CH-Ar), 122.98 (CH-Ar), 119.530 (aromatic. C), 113.79 (aromatic. C), 110.92 (quat. C), 60.82 (OCH2), 28.39 (CH2), 15.59 (CH3), 14.29 (CH3);GCMS m/z (EI, + ve): found 275.10 ([M + H]+), C15H18N2O3 calculated 275.14.
Ethyl 4-((4-Methoxyphenyl) Amino)-5-Oxo-2,5-Dihydro-1H-Pyrrole-3-Carboxylate (5c)
Light yellow solid; 25.05%; m.p. 70-71○C. IR (ATR) n/cm-1: 3327 (NH), 2982 (NH, amide), 1766 (C=O, ester), 1638 (C=C), 1517 (N-C=O), 755 (NH-Ar-OMe); 1H NMR (400 MHz, CD3COCD3) 8.79 (d, J = 10.9 Hz, 2H), 8.54 (d, J = 11.2 Hz, 2H), 6.14 (s, 2H), 5.30 (q, J = 8.9 Hz, 2H), 4.73 (s, 3H), 1.59 (t, J = 8.9 Hz, 3H); 13C NMR (100 MHz, CD3COCD3) δ 178.90 (C=O), 166.36 ( C=O), 159.38 (aromatic. C), 157.52 (quat. C), 143.66 (aromatic. C), 130.63 (quat. C), 125.11 (CH-Ar), 113.93 (quat. C), 67.56 (OCH2), 60.79 (CH2), 55.54 (OCH3), 14.35 (CH3); GCMS m/z (EI, + ve): found 277.10 ([M + H]+), C14H16N2O4 calculated 277.12.
General Procedure for the Synthesis of Pyrrolidine-Based Iminosugar Intermediates (6a-e).
Mixtures consisting 1 eq 6a-e were stirred in ethanol with Pd-C (10% wt.) (0.5 equiv.) and acetic acid (2 equiv.). The reactions were stirred vigorously under hydrogen atmosphere towards completion (24 h) and then filtered through Celite. After removal of the solvent, the crude products were used in following steps without further purification
Ethyl 4-Hydroxy-5-Oxopyrrolidine-3-Carboxylate (6a)
Colourless oily; 58%; IR (ATR) n/cm-1: 3392 (OH), 2915 (NH, amide), 1779 (C=O, ester), 1470 (CH2), 1352 (CH3); 1H NMR (400 MHz, CDCl3) δ 4.62 (d, J = 7.8 Hz, 1H), 4.55 (d, J = 12.0 Hz, 1H), 4.44 – 4.25 (m, 1H), 4.23 (t, J = 10.7 Hz, 2H), 3.65 – 3.25 (m, 1H), 1.28 (t, J = 7.1 Hz, 3H; 13C NMR (100 MHz, CDCl3) δ 154.58 (C=O), 145.25 (C=O), 68.38 (CHOH), 66.39 (OCH2), 62.10 (CH2), 45.61 (CH), 14.17 (CH3); GCMS m/z (EI, + ve): found 174.00 ([M + H]+), C7H11NO4 calculated 174.08.
Ethyl 4-Hydroxy-1-Methyl-5-Oxopyrrolidine-3-Carboxylate (6b)
White solid; 98%; m.p. 116-118○C. IR (ATR) n/cm-1: 3217 (OH), 2989 (NH, amide), 1721.73 (C=O, ester), 1503 (N-C=O), 1463 (CH2), 1353 (CH3); 1H NMR (400 MHz, CD3OD): δ 4.44 (d, J = 7.8 Hz, 1H), 4.15 (q, J = 7.2 Hz, 2H), 3.64 (dd, J = 10.1, 3.2 Hz, 1H), 3.28 (t, J = 1.6 Hz, 1H), 3.05 (s, 1H), 2.84 (d, J = 5.5 Hz, 3H), 1.28-1.20 (m, 3H); 13C NMR (100 MHz, CD3OD): δ 172.93 (C=O), 170.711 (C=O), 70.27 (CHOH), 60.71 (OCH2), 47.88 (CH2), 43.46 (CH), 28.70 (CH3-N), 13.15 (CH3); Anal. Calcd. for C8H13NO4: C, 51.33; H, 7.00; N, 7.48; O, 34.19. Found: C, 43.95; H, 6.13; N, 5.86; GCMS m/z (EI, + ve): found 187.10 ([M]+), C8H13NO4 calculated 187.08.
Ethyl 5-Oxo-4-(Phenylamino)Pyrrolidine-3-Carboxylate (6c)
Light yellow solid; 31.80%; m.p. 97-99○C. IR (ATR) n/cm-1: 3345.19 (NH), 3007 (NH, amide), 2985 (CH, aromatic) 1769.48 (C=O, ester), 1514 (N-C=O), 1470 (CH2), 1305 (CH3), 751 (NH-Ar); 1H-NMR (400 MHz, CDCl3): δ 7.21 (qd, 2H), 6.80 (qt, 1H), 6.67 (d, J = 7.3 Hz, 2H), 4.59 (dd, J = 9.6, 0.9 Hz, 1H), 4.45-4.36 (m, 2H), 4.14-4.00 (m, 2H), 3.81-3.73 (m, 1H), 1.05 (t, J = 7.3 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 174.19 (C=O), 170.24 (C=O), 146.25 (aromatic. C), 129.43 (CH-Ar), 119.40 (quat. C), 113.70 (CH-Ar), 66.99 (OCH2), 61.62 (CH2), 55.41 (CH), 46.17 (CH), 13.95 (CH3); GCMS m/z (EI, + ve): found 249.10 ([M + H]+), C13H16N2O3 calculated 249.12.
Ethyl 4-((4-Ethylphenyl)Amino)-5-Oxopyrrolidine-3-Carboxylate (6d)
Reddish yellow solid; 23.54%; m.p. 81-83○C. IR (ATR) n/cm-1: 3345.10 (NH), 3007.85 (NH, amide), 2980 (CH, aromatic), 1770.48 (ester), 1514 (N-C=O), 1452 (CH2), 1376 (CH3), 690 (NH-Ar); 1H-NMR (400 MHz, CDCl3): 7.04 (d, J = 8.7 Hz, 2H), 6.62 (d, J = 8.2 Hz, 2H), 4.60 (d, J = 10.1 Hz, 1H), 4.42 (dd, J = 10.1, 5.9 Hz, 1H), 4.34 (q, J = 4.1 Hz, 1H), 4.10 (qd, J = 7.1, 2.6 Hz, 2H), 3.78-3.72 (m, 1H), 2.54 (q, J = 7.6 Hz, 2H), 1.18 (q, J = 7.3 Hz, 3H), 1.08 (t, J = 7.3 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 174.20 (C=O), 170.17 (C=O), 144.16 (aromatic. C), 135.38 (aromatic. C), 128.75 (CH-Ar), 113.87 (CH-Ar), 66.93 (OCH2), 61.62 (CH2), 55.85 (CH), 46.37 (CH), 28.05 (CH2), 16.01 (CH3), 14.00 (CH3); GCMS m/z (EI, + ve): found 277.10 ([M + H]+), C15H20N2O3 calculated 277.15.
Ethyl 4-((4-Methoxyphenyl)Amino)-5-Oxopyrrolidine-3-Carboxylate (6e)
Yellow solid; 32.5%; m.p. 102-104○C. IR (ATR) n/cm-1: 3345 (NH), 3007 (NH, amide), 2980 (CH, aromatic), 1769 (C=O), ester), 1514 (N-C=O), 1460 (CH2), 1376 (CH3), 819 (NH-Ar); 1H-NMR (400 MHz, CDCl3): δ 6.79 (dd, J = 6.9, 2.3 Hz, 2H), 6.68-6.63 (m, 2H), 4.60 (d, J = 10.1 Hz, 1H), 4.41 (dd, J = 10.1, 5.9 Hz, 1H), 4.30 (q, J = 4.1 Hz, 1H), 4.15-4.04 (m, 3H), 3.74 (d, J = 3.7 Hz, 3H), 1.09 (t, J = 7.1 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 174.30 (C=O), 172.22 (C=O), 153.44 (aromatic. C), 140.32 (aromatic. C), 115.27 (CH-Ar), 114.13 (CH-Ar), 66.91 (OCH2), 61.62 (CH2), 56.46 (CH), 55.79 (OCH3), 46.38 (CH), 14.02 (CH3); GCMS m/z (EI, + ve): found 279.10 ([M + H]+), C14H18N2O4 calculated 279.13.
General Procedure for the Synthesis of Pyrrolidine-Based Iminosugars Intermediates (8a-e)
A stirred mixture of 4c-g (1 eq.) in CH2Cl2 (0.05 L) was added acetic acid (1 eq.) and NaBH4 (1.1 mol) at 0oC. After 0oC achieved, the mixture was stirred for one hour, then the mixture was stirred at room temperature upon completion (8 h). The solvent was then removed from mixtures and was extracted with EtOAc. The organic layer was washed with highly saturated NaHCO3 solution. Trace of water was removed with anhydrous MgSO4 and concentrated in vacuo. The crude product was purified by column chromatography to give the hydroxy ester product (8a-E).
Ethyl-2-Ethyl-4-Hydroxy-5-Oxopyrrolidine-3-Carboxylate(8a)
White solid; 99%; m.p. 112-114○C. IR (ATR) n/cm-1: 3312 (OH), 3184 (NH, amide), 1709 (C=O, ester) 1459 (CH2), 1371 (CH3); 1H-NMR (400 MHz, CD3OD): δ 4.47 (t, J = 9.8 Hz, 1H), 4.22 (s, 2H), 3.60 (s, 1H), 2.71 (d, J = 11.0 Hz, 1H), 1.74-1.58 (m, 2H), 1.35-1.23 (m, 3H), 0.95 (dd, J = 18.5, 7.5 Hz, 3H); 13C NMR (100 MHz, CD3OD) δ 175.40 (C=O), 172.19 (C=O), 72.74 (CHOH), 61.12 (OCH2), 54.76 (CH), 54.42 (CH), 27.89 (CH2), 13.17 (CH3), 8.22 (CH3); LCMS m/z (H-ESI, + ve): found 202.1072 ([M+H]+), C9H15NO4 calculated 202.1073.
Ethyl-4-Hydroxy-2-Isopropyl-5-Oxopyrrolidine-3-Carboxylate(8b)
White solid; 99%; m.p. >175○C decomposed. IR (ATR) n/cm-1: 3366 (OH), 2971 (NH, amide), 1726 (C=O, ester), 1682 (N-C=O), 1466 (CH2), 1375 (CH3); 1H-NMR (400 MHz, CD3OD) δ 4.44-4.37 (1H), 4.23-4.11 (2H), 3.55-3.48 (1H), 3.34-3.31 (1H), 2.80-2.71 (1H), 1.85-1.74 (1H), 1.30-1.22 (3H), 0.98-0.82 (6H); 13C NMR (100 MHz, CD3OD): δ 175.28 (C=O), 172.80 (C=O), 73.23 (CHOH), 61.12 (OCH2), 58.83 (CH), 52.39 (CH), 31.81 (CH), 16.77 (CH3x2), 13.09 (CH3); LCMS m/z (H-ESI, + ve): found 216.1228 ([M+H]+), C10H17NO4 calculated 216.1230.
Ethyl-4-Hydroxy-5-Oxo-2-(p-Tolyl)Pyrrolidine-3-Carboxylate(8c)
White solid; 94.44%; m.p. 189-200○C. IR (ATR) n/cm-1: 3294 (OH), 3054 (NH, amide), 2981 (CH, aromatic), 1729 (C=O, ester), 1518 (N-C=O), 1458 (CH2), 642 (Ar-CH3); 1H-NMR (400 MHz, CD3OD): δ 7.25-7.15 (4H), 4.66-4.63 (1H), 4.58-4.53 (1H), 4.22-4.04 (2H), 2.94-2.84 (1H), 2.35-2.26 (3H), 1.21-1.14 (3H); 13C NMR (100 MHz, CD3OD): δ 175.47 (C=O), 171.42 (C=O), 138.24 (aromatic. C), 136.98 (aromatic. C), 127.68 (CH-Ar), 126.19 (CH-Ar), 72.69 (CHOH), 61.08 (CH), 59.08 (CH), 56.53 (CH), 19.82 (CH3), 13.12 (CH3); GCMS m/z (EI, + ve): found 263.10 ([M]+), C14H17NO4 calculated 263.12.
Ethyl-2-(4-Cyanophenyl)-4-Hydroxy-5-Oxopyrrolidine-3-Carboxylate(8d)
White solid; 82.73%; m.p. >231○C decomposed. IR (ATR) n/cm-1: 3200 (OH), 1675 (C=O, ester), 1476 (CH2), 637 (Ar-CN); 1H-NMR (400 MHz, CD3OD): δ 7.75 (d, J = 8.2 Hz, 2H), 7.54 (d, J = 8.2 Hz, 2H), 4.80 (d, J = 8.2 Hz, 1H), 4.58 (d, J = 9.1 Hz, 1H), 4.17 (t, J = 7.3 Hz, 2H), 2.88 (t, J = 8.7 Hz, 1H), 1.20 (t, J = 7.1 Hz, 3H); 13C NMR (100 MHz, CD3OD): δ 175.54 (C=O), 175.10 (C=O), 152.07 (aromatic. C), 132.48 (CH-Ar), 129.96 (CH-Ar), 127.45 (CN), 110.78 (aromatic. C), 73.92 (CHOH), 58.84 (OCH2), 43.97 (CH), 42.24 (CH), 11.85 (CH3); GCMS m/z (EI, + ve): found 297.00 ([M + Na]+), C14H14N2O4 calculated 297.08.
Ethyl-4-Hydroxy-2-(4-Methoxyphenyl)-1-Methyl-5-Oxopyrrolidine-3-Carboxylate(8e)
Yellow solid; 99%; m.p. 54-55○C. IR (ATR) n/cm-1: 3217 (OH) , 2989 (CH, aromatic), 1721 (C=O, ester), 1503 (N-C=O), 1473 (CH2), 688 (Ar-OMe) ; 1H-NMR (400 MHz, CD3OD): δ 7.19 (d, J = 8.7 Hz, 2H), 6.89 (d, J = 8.7 Hz, 2H), 4.80-4.75 (m, 1H), 4.55 (d, J = 7.3 Hz, 1H), 3.84-3.70 (m, 5H), 3.62 (t, J = 7.1 Hz, 1H), 2.69 (d, J = 7.3 Hz, 3H), 0.90 (t, J = 7.1 Hz, 3H); 13C NMR (100 MHz, CD3OD) δ 169.07 (C=O), 168.04 (C=O), 160.06 (aromatic. C), 129.51 (CH-Ar), 126.86 (aromatic. C), 113.55 (CH-Ar), 70.30 (CHOH), 61.83 (CH) , 60.22 (OCH2), 54.42 (OCH3) , 51.32 (CH), 27.92 (CH3-N), 12.79 (CH3); Anal. Calcd. for C14H17NO5: C, 60.21; H, 6.14; N, 5.02; O, 28.64. Found: C, 57.68; H, 2.84; N, 6.59; GCMS m/z (EI, + ve): found 295.10 ([M + 2H]+), C15H19NO5 calculated 295.14.
General Procedure for the Synthesis of Pyrrolidine-Based Iminosugars (7a-e) and (9a-e)
The crude products obtained were dissolved in dry tetrahydrofuran and were added slowly to the lithium aluminium hydridesolution (excess) in inert atmosphere. The mixtures were heated at 90 oC towards completion (4-8 h) and then cooled to 0 °C. The reaction mixtures were quenched by adding of distilled water, and the mixtures were filtered through Celite and concentrated in vacuo to give the crude products iminosugar derivatives. Compounds (7a-e) and (9a-e) as oil after purification by column chromatography.
4-(Hydroxymethyl)Pyrrolidin-3-ol (7a)
Reddish oily; 81.23%; IR (ATR) n/cm-1: 3278 (CH-OH), 2927 (NH, stretch), 1654 (NH, bend), 1412 (CH2), 1016 (C-O); 1H-NMR (400 MHz, CD3OD): δ 3.81-3.72 (m, 2H), 3.67 (q, J = 5.6 Hz, 3H), 3.58 (td, J = 11.5, 5.5 Hz, 2H), 1.78 (q, J = 5.6 Hz, 1H); 13C NMR (100 MHz, CD3OD): δ 71.30 (CHOH), 64.32 (CH2), 60.28 (CH2OH), 59.69 (CH), 45.75 (CH); LCMS m/z (ESI-QTOF, + ve): found 119.0794 ([M + 2H]+), C5H11NO2 calculated 119.0941.
4-(Hydroxymethyl)-1-Methylpyrrolidin-3-ol (7b)
Reddish oily; 64.27%; IR (ATR) n/cm-1: 3254 (OH), 2948 (NH, stretch), 1654 (NH, bend), 1407 (CH2), 1021 (C-O); 1H NMR (400 MHz, CD3OD): δ 4.36 (td, J = 6.1, 3.8 Hz, 1H), 3.77 (dd, J = 11.0, 6.4 Hz, 1H), 3.60 (dd, J = 11.0, 7.3 Hz, 1H), 3.05 (q, J = 5.5 Hz, 1H), 2.84 (dd, J = 9.6, 7.8 Hz, 1H), 2.53-2.43 (m, 2H), 2.38 (s, 3H), 1.90-1.80 (m, 1H); 13C NMR (100 MHz, CD3OD): δ 71.17 (CHOH), 63.87 (CH2), 60.17 (CH2OH), 57.42 (CH2), 45.31 (CH3-N), 41.56 (CH); LCMS m/z (ESI-QTOF, + ve): found 132.1044 ([M + H]+), C6H13NO2 calculated 132.1019.
(4-(Phenylamino) Pyrrolidin-3-ol) (7c)
Yellow solid; 22.19%; IR (ATR) n/cm-1: 3345 (OH), 2917 (NH, stretch), 2830 (CH, aromatic), 1599 (NH, bend), 1496 (CH2), 1020 (C-O), 692 (NH-Ar); 1H-NMR (400 MHz, CD3OD) : δ 7.05 (dd, J = 8.7, 7.3 Hz, 2H), 6.64 (dd, J = 7.8 Hz, 2H), 6.55 (tt, J = 7.1 Hz, 1H), 3.75 (dd, J = 5.5, 3.7 Hz, 2H), 3.70 (d, J = 5.9 Hz, 2H), 3.66 (s, 3H), 2.02-1.93 (m, 1H); 13C NMR (100 MHz, CD3OD): δ 148.39 (aromatic. C), 128.76 (CH-Ar), 116.50 (CH-Ar), 113.04 (CH-Ar), 61.37 (CH2OH), 60.05 (CH2), 53.76 (CHNH), 44.67 (CH), 29.43 (CH2); LCMS m/z (ESI-QTOF, + ve): found 194.1199 ([M + 2H]+), C11H16N2O calculated 194.1414.
(4-((4-Ethylphenyl) Amino) Pyrrolidin-3-ol) (7d)
Reddish oily; 38.5%; IR (ATR) n/cm-1: 3243.34 (OH), 2915 (NH, stretch), 2845 (CH, aromatic), 1518 (NH, bend), 1455 (CH2) 1019 (C-O), 820 (NH-Ar); 1H-NMR (400 MHz, CD3OD): δ 6.91 (d, J = 8.7 Hz, 2H), 6.59 (d, J = 8.7 Hz, 2H), 3.79-3.72 (m, 2H), 3.70 (d, J = 5.9 Hz, 2H), 3.65 (d, J = 3.7 Hz, 3H), 2.46 (q, J = 7.6 Hz, 2H), 1.98 (t, J = 5.5 Hz, 1H), 1.12 (t, J = 7.5 Hz, 3H); 13C NMR (100 MHz, CD3OD): δ 146.20 (aromatic. C), 132.72 (aromatic, C), 128.08 (CH-Ar), 113.50 (CH-Ar), 61.39 (CH2OH), 60.28 (CH2), 60.06 (CH2), 54.30 (CHNH), 44.66 (CH), 27.65 (CH2), 15.32 (CH3); LCMS m/z (ESI-QTOF, + ve): found 222.1514 ([M + 2H]+), C13H20N2O calculated 222.1727.
(4-((4-Methoxyphenyl) Amino)Pyrrolidin-3-ol) (7e)
Brown solid; 70.28%; m.p. 84○C. IR (ATR) n/cm-1: 3252 (OH), 2912 (NH, stretch), 2830 (CH, aromatic), 1508 (NH, bend), 1462 (CH2), 1033 (C-O), 823 (NH-Ar); 1H-NMR (400 MHz, CD3OD): δ 6.71 (dd, J = 6.4, 2.3 Hz, 2H), 6.67-6.62 (m, 2H), 3.80-3.72 (m, 2H), 3.71-3.68 (m, 2H), 3.67 (s, 3H), 3.64 (d, J = 5.5 Hz, 2H), 3.55 (q, J = 5.0 Hz, 1H), 1.97 (q, J = 5.6 Hz, 1H); 13C NMR (100 MHz, CD3OD): δ 61.17 (CH2), 60.44 (CH2), 60.14 (CH2) 55.41 (CH), 54.85 (CH), 44.66 (CH); LCMS m/z (ESI-QTOF, + ve): found 224.1326 ([M + 2H]+), C12H18N2O2 calculated 224.1519.
5-Ethyl-4-(Hydroxymethyl)Pyrrolidin-3-ol (9a)
Yellow oily; 99%; IR (ATR) n/cm-1:3250 (OH), 1549 (NH, bend), 1410 (CH2), 1345 (CH3), 1019 (C-O); 1H-NMR (400 MHz, CD3OD): δ 4.19-4.13 (m, 1H), 3.62-3.49 (m, 2H), 3.29-3.27 (m, 1H), 3.02-2.83 (m, 3H), 2.06-1.89 (m, 2H), 1.00 (t, J = 7.5 Hz, 3H); 13C NMR (100 MHz, CD3OD): δ 73.75 (CHOH), 62.57 (CH), 61.20 (CH2), 55.32 (CH), 52.78 (CH2OH), 27.19 (CH2), 10.30 (CH3); LCMS m/z (ESI-QTOF, + ve): found 146.1161 ([M + H]+), C7H15NO2 calculated 146.1175.
4-(Hydroxymethyl)-5-Isopropylpyrrolidin-3-ol (9b)
Yellow oily; 99%; IR (ATR) n/cm-1: 3293 (OH), 2919 (NH, stretch), 2850 (CH), 1506 (NH, bend), 1408 (CH2), 1019 (C-O); 1H-NMR (400 MHz, CD3OD): δ 4.27 (s, 1H), 3.61 (dd, J = 11.0, 4.6 Hz, 1H), 3.45 (dd, J = 11.0, 6.4 Hz, 1H), 3.10 (d, J = 3.2 Hz, 2H), 2.89 (dd, J = 8.7, 6.4 Hz, 1H), 2.15-2.00 (m, 1H), 1.97-1.89 (m, 1H), 1.03 (t, J = 6.9 Hz, 6H); 13C NMR (100 MHz, CD3OD): δ 73.24 (CHOH), 61.67 (CH2), 52.73 (CH), 52.54 (CH2OH), 31.70 (CH), 18.80 (CH3), 18.69 (CH3); LCMS m/z (ESI-QTOF, + ve): found 160.1315 ([M + H]+), C8H17NO2 calculated 160.1332.
4-(Hydroxymethyl)-5-(p-Tolyl)Pyrrolidin-3-ol (9c)
Yellow solid; 68.27%;m.p. exceed 270○C decomposed. IR (ATR) n/cm-1:3676 (OH), 3565 (NH, stretch), 2850 (CH, aromatic), 1574 (NH, bend), 1420 (CH2), 843 (Ar-CH3), 1H-NMR (400 MHz, CD3OD): δ 7.28 (d, J = 8.2 Hz, 2H), 7.13 (d, J = 7.8 Hz, 2H), 4.28 (s, 1H), 3.72 (d, J = 7.8 Hz, 1H), 3.62 (s, 1H), 3.49 (s, 1H), 2.97 (d, J = 13.3 Hz, 2H), 2.30 (d, J = 4.6 Hz, 3H), 2.11-2.00 (1H); 13C NMR (100 MHz, CD3OD ) δ 140.71 (aromatic. C), 135.60 (aromatic. C), 128.88 (CH-Ar), 126.56 (CH-Ar), 72.10 (CHOH), 66.90 (CH), 57.00 (CH2), 56.20 (CHOH), 48.31 (CH), 21.3 (CH3); LCMS m/z (ESI-QTOF, + ve): found 208.1308 ([M + H]+), C12H17NO2 calculated 208.1332.
4-(4-Hydroxy-3-(Hydroxymethyl)Pyrrolidin-2-yl)Benzonitrile (9d)
Brown oily; 67.84%;IR (ATR) n/cm-1: 3319 (OH), 2915 (NH, stretch), 2848 (CH, aromatic), 1636.93 (NH, bend), 1420 (CH2), 1018 (C-O), 606 (Ar-CN); 1H-NMR (400 MHz, CD3OD): δ 7.29 (d, J = 8.2 Hz, 2H), 7.14 (d, J = 7.8 Hz, 2H), 4.35-4.25 (m, 1H), 3.78 (d, J = 8.2 Hz, 1H), 3.64 (dd, J = 11.2, 4.8 Hz, 1H), 3.48 (q, J = 5.6 Hz, 1H), 2.99 (t, J = 5.3 Hz, 2H), 2.09 (s, 1H); 13C NMR (100 MHz, CD3OD): δ 138.41 (aromatic. C) 137.18 (CN) 128.98 CH-Ar), 127.13 (CH-Ar), 126.46 (aromatic. C) 74.53 (CHOH), 64.57 (CH), 60.40 (CH2), 58.35 (CH), 54.21 (CH2OH); LCMS m/z (ESI-QTOF, + ve): found 222.1350 ([M+ 4H]+), C12H14N2O2 calculated 222.1363
4-(Hydroxymethyl)-5-(4-Methoxyphenyl)-1-Methylpyrrolidin-3-ol (9e)
Brown oily;32.33%;IR (ATR) n/cm-1: 3319 (OH), 1560 (NH, bend), 1408 (CH2), 1026 (C-O), 628 (Ar-OMe); 1H-NMR (400 MHz, CD3OD): δ 7.42 (d, J = 9.1 Hz, 2H), 6.92 (d, J = 9.1 Hz, 2H), 4.61-4.52 (m, 1H), 4.13 (d, J = 10.1 Hz, 1H), 3.78 (s, 3H), 3.55 (dd, J = 11.0, 8.7 Hz, 1H), 3.44 (d, J = 11.0 Hz, 1H), 3.14 (q, J = 5.3 Hz, 1H), 3.05 (dd, J = 11.0, 4.1 Hz, 1H), 2.76-2.64 (m, 1H), 2.55 (s, 3H); 13C NMR (100 MHz, CD3OD): δ 178.49 (aromatic. C), 160.19 (aromatic. C), 130.54 (CH-Ar), 113.68 (CH-Ar), 72.50 (CHOH), 69.38 (CH), 62.88 (CHOH), 58.20 (CH2OH), 54.89 (OCH3), 48.68 (CH), 39.10 (CH3-N); LCMS m/z (ESI-QTOF, + ve): found 238.1499 ([M + H]+), C13H19NO3 calculated 238.1438.
Determination of Alpha-Glucosidase Inhibition Activity
The alpha-glucosidase enzyme inhibitory activity assay was adopted from the method described by Lee et al. with slight modification14. The inhibition rate of alpha glucosidase was determined at 37◦C in phosphate buffer solution (pH 6.5). The reaction mixtures containing 10 μl of sample, 20 μl of alpha glucosidase enzyme, 20 μl of water and 40 μl of buffer solution were mixed in microtiter plate. The mixtures were pre-incubated at 37°C for 10 minutes. Then, 10 μl of substrate in buffer solution were added, and the incubation time was prolonged to an additional 30 minutes. The absorption at 405 nm was measured instantly after 30 minutes of incubation with microplate spectrophotometer and the inhibition activity was calculated by the equation described by Lee et.al14.
Results and Discussion
Synthesis of Pyrrolidine-Based Iminosugar Derivatives
The synthetic strategy used in this study is outlined in Scheme 1. The pyrrolidine based iminosugar derivatives were synthesized in three or four steps linear synthetic route (Scheme 1) starting with the multicomponent reaction (MCR), amination, reduction of olefinic bond followed by simultaneous reductions of both the ester and amide (for compounds 7c-e using 4 steps strategy) (Scheme 1).
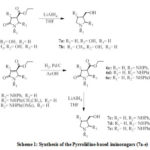 |
Scheme 1: Synthesis of the Pyrrolidine-based iminosugars (7a-e) |
According to Mohammat et al., the 2,3-dioxopyrolidine skeleton 4 can be obtained through a multicomponent reaction by reacting sodium diethyl oxaloacetate with the same molar concentrations of aldehydes 3 and amines 2 at reflux condition in moderate yields7 (Scheme 2).
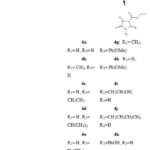 |
Scheme 2: Synthesis of 2, 3-dioxopyrrolidines via a multicomponent reaction |
The crude product was then acidified and filtered to give the desired products, 4a-k. However, for the synthesis of compound 4a an excess of amount of ammonia is required15. The amination of compound 2,3-dioxopyrrolidines 4a of the keto group at C-3 gave derivatives of different 4-hydroxy-5-oxo-2,5-dihydro-1H-pyrrole-3-carboxylic acid esters 5a-c16. These attempts were refluxed in ethanol for 12 and 24 hours but the yield obtained were low (21-26%). Jourdan et al., reported similar observation during the amination of 4-hydroxy-5-oxo-2,5-dihydro-1H-pyrrole-3-carboxylic acid esters molecule17,16,18. The formic acid was added to favor the 4a in keto form19, however the yields 5a-c were similar .
Subsequently, compounds 5a-c were treated with acetic acid in the presence of Pd/C catalyst which underwent high diastereoselective hydrogenation at the olefenic bond of the pyrrolidine skeleton yielding 6a-e as cis-configured isomers8. Reductions of the enolic tautomers 4a and 4b by hydrogenation were performed under neutral or acidic conditions in ethanol for 3 hours. The mechanism of the catalytic reduction of these compounds was proposed by Mohammat 20158. This strategy furnished the cis-hydroxy esters 6a and 6b in with moderate to high yields 58% and 98%, respectively8. Similarly, stereoselective reduction of enamine tautomers 5a-c via syn hydrogenation was also performed under neutral condition for 24 hours but the yields obtained were low (31-36%). Adding acetic acid as the catalyst to the reaction condition reduced the reaction time, but the yields were not improved20,21,22,23.
The catalytic hydrogenation of 5a-c is formed via the chelation ring formation as proposed by Harada and Matsumoto24. The six-membered chelated ring structure with the catalyst is present in the intermediate, the hydrogen atom preferentially attacked from the less hindered side to afford compounds 6c-e24. The reduction of enamine was found to be low in yield under acidic and neutral conditions due to the presence of nitrogen atom of the amine derivatives which poisoned the palladium catalyst and degraded the selectivity of the catalyst25. These reactions were caused by the electron donor of N-H group of an amine derivatives, which has the capability to compete for coordination at the catalytic site25. Similar observation reported by Xie et al., during hydrogenation of enamines and imines26. Compounds 7a-e were obtained by reduction of amide and ester via excess LiAlH4 to give the target molecule27.
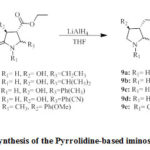 |
Scheme 3: Synthesis of the Pyrrolidine-based iminosugars (9a-e) |
Alternatively, 2,3-dioxo-4-carboxy-pyrrolidines (4c-g) underwent stereoselective reduction using NaBH4/AcOH to specifically yield8a-e as trans-configured analogues in good yields. The mechanism and steric effect in this type of reduction reaction was proposed by Mohammat 2015 as shown in Figure 48. It was predicted throughout the reaction, compounds 4 tautomerized into both the enol and keto forms; however the keto analogues are more stable due to reaction being catalyzed by an acid. In terms of diastereoselection, the bulky C-5 substituent noticeably contributed towards the steric factor and this gave only the thermodynamically-stable trans product (8a-e)8. Therefore, the hydride was transfered from the less hindered part of P, away
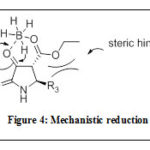 |
Figure 4: Mechanistic reduction |
from the C-5 substituent forcing the hydroxy group to be in trans-position to that of the ethyl ester8. Then, the amide and ester functionalities of compounds 8a-e were reduced further with an excess LiAlH4 to give compounds 9a-e in moderate to high yields.
Biological Activity of Pyrrolidine-Based Iminosugar Derivatives
Ten iminosugars and their respective intermediates were synthesized to evaluate their α-glucosidase inhibitory activity in comparison with deoxynojirimycin (DNJ) as the positive control, as shown in Table 1. The inhibition percentages against glucosidase of these were tested on two different concentrations at 1.0 mM and 5.0 mM. The inhibition percentages in 1.0 mM and 5.0 mM of DNJ were found to be 59.38±1.22 and 80.75±0.38, respectively and it was regarded as a positive control. In general, the present or increase in the inhibition activity is closely associated with the aryl subtituents bearing either electron donating groups (-OMe and CH3) or an electron withdrawing group (-CN). Among the ten synthesized iminosugars, 7e gave the highest inhibition of 67.5±0.5 at 1.0 mM followed by 9c with 17.9 ± 3.5 and 7c with 4.3±0.7. Compound 7e gave better inhibition activity at 1.0 mM concentration towards glucosidase as compared to DNJ. The presence of methoxy-containing arylamine at C-3 position somehow contributes towards the formation of iminosugars as potential glucosidase inhibitors. Further modification at the pyrrolidine skeleton is thus to enhanced the bio activity of the imino sugar while maintaining the presence of the p-methoxy arylamine at the C-3 position.
Table1: Alpha-Glucosidase inhibition studies of synthesize compunds
|
Iminosugars
|
Alpha-Glucosidase Inhibition (%) |
|
|
1.0mM |
5.0mM |
|
|
5b |
NT |
NT |
|
6e |
NT |
NT |
|
7a |
NI |
NI |
|
7b |
NI |
NI |
|
7c |
4.3 ± 0.7 |
16.2 ± 1.1 |
|
7d |
NI |
5.7±0.7 |
|
7e |
67.5 ± 0.5 |
NI |
|
9a |
NI |
NI |
|
9b |
NI |
NI |
|
9c |
17.9 ± 3.5 |
80.9 ± 0.3 |
|
9d |
NI |
16.5 ± 1.8 |
|
9e |
NI |
NI |
|
DNJ |
59.38±1.22 |
80.75±0.38 |
% Inhibition determined at 1.0 mM and 5.0 mM concentration of compound
NI: No Inhibition, NT: Not Tested
Conclusion
In conclusion, ten pyrrolidine-based iminosugars were synthesized in three or four steps utilizing MCR as the key step. One compound, 7e (4-((4-methoxyphenyl)amino)pyrrolidin-3-ol) demonstrated a strong potent inhibitory activity at 1.0 mM concentration against alpha-glucosidase test. In addition, the presence of a methoxy group at C-3 plays an important role in the inhibition and that further structural variations at the nitrogen atom of the skeleton using different amine derivatives could improve the alpha glucosidase activity.
Acknowledgments
The authors wish to thank the Institute of Science (IOS), UiTM Shah Alam, Malaysia for its generous support and the Ministry of Education (MOE), Malaysia for the financial support under the Fundamental Research Grant Scheme (600-IRMI/FRGS 5/3 (109/2019).
Conflict of Interests
The authors declare that there is no conflict of interests related to the publication of this paper.
Refferences
- Prasad, P.; Surajit, M.; Sudhir, K. T.; Santosh, A., Eur. J. Nutr., 2015.
- Sivaprasad, K.; Sujatha, S.; Srinivas, U.; Jaya, S. A.; Shubham D.; Hasitha, S. A.; Yogeeswari, P.; Dilep K. S.; Bathini N. B.; Krishna, S. E., Bioorg., Med. Chem. Lett., 2017, 27, 2818–2823.
- Clifford, J. B.; Caroline, D., Br J Cardiol., 2003, 10, 128-36.
- Lucassen, P.; Rutten, G.; Lucassen, C. V. W.; Rutten, P.; Van, G. W. C., Cochrane Database Syst. Rev., 2005, 2.
- Erica, C. S.; Nathália, C. G. Y.; Alcindo, A. D. S.; Fernando, C. R., Synthesis, 2017, 49, 4869–4875.
- Doddi, V. R.; Yashwant D. V., European J. Org. Chem., 2007, 33, 5583–5589.
- Mohammat, M. F.; Shaameri, Z.; Hamzah, A. S., Molecules, 2009, 14(1), 250–256.
- Mohammat, M. F.; Mansor, N.S.; Shaameri, Z.; Hamzah, A. S., J. Korean Chem. Soc, 2015, 59 (1), 31–35.
- Zhang, E.; Bai, P. Y.; Sun, W.; Wang, S.; Wang, M. M.; Xu, S. M., Carbohydr. Res., 2016, 434, 33–36.
- Kotkar, S. P.; Chavan, V. B.; Sudalai, A., Org. Lett., 2007, 9(6) 1001-1004.
- Masakazu, S.; Zhangyong, H.; Liang, P. H.; Stephen, M. D.; Lisa, J. W. H.; William, A. G.; Chi-Huey., J. Am. Chem. Soc.,2007, 6, 14811–14817.
- En-Lun, T.; Sih-Yu, C.; Ming-Hsun, Y.; Shih-Chi, W.; Ting-Ren, R. C., Wei-Chieh, C., Bioorg. Med. Chem., 2008, 16 (24), 10198–10204.
- Shaameri, Z.; Ali, S. H. S.; Mohamat, M. F.; Yamin, B. M.B.; Hamzah, A. S., J. Heterocycl. Chem., 2009, 0, 1208–1212.
- Lee, S.; Lin, H.; Chen, C., Phytochemistry, 2008, 69, 2347–2353.
- Metten, B.; Kostermans, M.; Baelen, G. V.; Smet, M.; Dehaen, W., Tetrahedron, 2006, 62 (25), 6018–6028.
- Southwick, P. L.; Hofmann, G.H., J. Org. Chem., 1963, 28(5), 1332–1336.
- Jourdan, F.; Kaiser, J. T.; Lowe, D. J., Synth. Commun., 2006, 35, 2453–2466.
- Augustin, M.; Jeschke, P., J. prak. Chem., 1987, 329, 599-606.
- Madhav, R.; Richard, F. D.; Southwick, P. L., J. Heterocycl. Chem., 1973, 10, 25-28.
- Ikemoto, N.; Tellers, D.M.; Dreher, S.D.; Liu, J; Huang, A.; Rivera, N. R.; Njolito, E.; Hsiao, Y.; Williams, J. C.; Williams, J.M.; Armstrong, J. D.; Yongkui, S.; Mathre, D. J.; Grabowski, E. J. J.; Tillyere, R. D., J. Am. Chem. Soc. 2004, 126 (10), 3048–3049.
- Richard F. B.; Mark D. B.; Durstl, H. D., J. Am. Chem. Soc., 1970, 3(1), 1968–1970.
- Smaliy, R. V.; Aleksandra A. C.; Nataliya A. S.; Sergey A. Y.; Aleksandr A. Y.; Aleksandr I. L.; Alina O. G.; Aleksandr N. K., J. Fluor. Chem., 2015, 180, 257–264.
- Tungler, B. A.; Tarnai, T.; Hegediis, L.; Fodor, K.; Mathe, T., Platinum Metals Rev., 1998, 42(3), 108–115.
- Mitsuru, F.; Tadashi, O.; Yoshihide, N.; Yuriko, T., Chem. Pharm. Bull., 1979, 27(9), 2223-2225.
- Marcazzan, P; Patrick, B. O.; James, B. R., Organometallics, 2003, 22 (6), 1177–1179.
- Xie, J. H.; Zhu, S. F.; Zhou, Q. L., Chem. Rev., 2011, 111(3), 1713–1760.
- Goti, A.; Cicchi, S.; Cacciarini, M.; Cardona, F.; Fedi, V.; Brandi, A., European J. Org. Chem., 2000, 21, 3633–3645.

This work is licensed under a Creative Commons Attribution 4.0 International License.









