Tetraethyllead (TEL) in Gasoline as a Case of Contentious Science and Delayed Regulation: A Short Review
Department of Chemistry, Hooghly Mohsin College, Chinsurah, Hooghly, West Bengal, India, PIN -712101
Corresponding Author E-mail: chhandasi_guharoy@yahoo.co.in
DOI : http://dx.doi.org/10.13005/ojc/360111
Article Received on : 25-01-2020
Article Accepted on :
Article Published : 10 Feb 2020
The article traces the history of tetraethyllead from its discovery as an antiknock agent in gasoline till its worldwide ban. It is worth revisiting how a much applauded discovery of science actually turned out to be a doom for mankind causing severe health hazards to generation after generation over a period of about 75 years. The article highlights the discovery, synthesis and antiknock properties of tetraethyllead, along with its severe toxic effects to human body which finally resulted in is ban. It also examines the phaseout and ban of tetraethyllead from the viewpoint of the Environmental Kuznets Curve. In addition, the article discusses the importance of prohibition of leaded gasoline from a social perspective. It puts forward the relation between the use of tetraethyllead and violence in the society. Also, it explores how tetraethyllead caused a drop in IQ level, especially among children.
KEYWORDS:Antiknock Properties; Phaseout and Ban; Social Perspective; Synthesis; Toxic Effects; Tetraethyllead in Gasoline.
Download this article as:| Copy the following to cite this article: Sarkar C. G. Tetraethyllead (TEL) in Gasoline as a Case of Contentious Science and Delayed Regulation: A Short Review. Orient J Chem 2020; 36(1). |
| Copy the following to cite this URL: Sarkar C. G. Tetraethyllead (TEL) in Gasoline as a Case of Contentious Science and Delayed Regulation: A Short Review. Orient J Chem 2020; 36(1). Available from: https://bit.ly/2SfK6nm |
Introduction
For nearly 75 years mankind had been subjected to the harmful effects of lead, generated from automobile fuels.1 Tetraethyllead was the chief antiknock agent used in gasoline for the major part of the 20th century which was the main source of lead toxicity in the environment.1,2,3 From its appearance as an antiknock agent in gasoline, in the 1920’s4, till its complete removal around the turn of this century,3 use of tetraethyllead remains a story of environmental degradation and health hazards that could have been avoided, but for commercial interests.1 The history of the growth and decline of leaded gasoline, thus should never be shelved but be maintained as a cautionary backdrop to aggressive and unregulated science. Indeed it is still relevant, since in 2017, the Nairobi Convention of the UNEP (United Nations Environment Programme) discussed the global picture of the use of leaded gasoline.5 Gasoline and the environmental impact of its use, as well as the properties of automobile exhausts constitute a wide area of research to this day.6, 7, 8
In the early twentieth century, the ongoing conflict between gasoline and ethanol seemed to be over. The contenders were the advocates of ethyl alcohol on one side and a group of automobile, chemical and oil companies on the other. For years, the battle continued, first over the fuel that would be best for the automobiles and then – after gasoline won the first hurdle – over what “anti-knock” substance would be added to gasoline. Oil won again, until new scientific studies raised concerns over the health hazards caused by lead additives in gasoline.
In the early days of the automobiles, ethanol was the major fuel worldwide and a burning question existed about what would fuel the future cars; gasoline or ethanol. Henry Ford was a big exponent of ethanol which he called the “The fuel of the future”. Ford stated “There is enough alcohol in one year’s yield of an acre of potatoes to drive the machinery necessary to cultivate the fields for 100 years.” 9,10
However, when in the early twentieth century, huge oil fields were discovered in Texas,11 oil became cheaper than ethanol. Over the period, as more and more wells were drilled and the price of gasoline reduced, it became the chief fuel to power the automobiles.
Then the battle moved in the direction to find out the most suitable substance, which when added to gasoline could reduce engine knocking.
Global Perspective
Engine Knocking
The modern internal combustion engine was successfully built by N.A. Otto in 1876.12
An internal combustion engine is in principle a heat engine where the combustion of a fuel takes place with an oxidizing agent (usually air) in a combustion chamber. The high-temperature and high-pressure gases that are produced due to combustion expand and apply direct force to some component of the engine namely pistons, turbine blades or rotors. This force then causes the component to move over a certain distance, converting chemical energy into useful mechanical energy. The static compression ratio of an internal combustion engine is the ratio of the volume of its combustion chamber from its largest capacity to its smallest capacity. The compression ratio is the measure of the efficiency of the engine; the higher the compression ratio, the greater the fuel economy and power output. However, when the compression ratio for a given fuel is too high, the engines are exposed to “knocking”.
Gasoline is the primary fuel used in internal combustion engines. It consists mostly of hydrocarbons (alkanes, alkenes and cycloalkanes) and is obtained by the fractional distillation of petroleum. These hydrocarbons have their own characteristic ignition temperature. When in a car engine the gasoline vapour-air mixture is compressed before sparking, some hydrocarbons tend to ignite under pressure before they are sparked. The shock waves thus created cause the characteristic metallic “pinging” sound. This is called ‘knocking’. Knocking causes loss of power, mechanical damage and overheating. Intense knocking can even break the piston or the engine. The use of gasoline as fuel in the early era of automobiles faced this severe challenge: to find a substance that would reduce knocking.
Anti-Knock Agents
An antiknock agent is an additive, which when added to a particular fuel reduces engine knocking and increases the fuel’s octane rating. Octane rating is a measure of a fuel’s ability to resist ‘knock’. The higher the octane–number the greater is the fuel’s resistance to knocking. The octane number of gasoline is the % of 2,2,4-trimethylpentane in a mixture with n-heptane (Fig. 1) that has the same knocking characteristics as that under test. 2,2,4-trimethylpentane (an isomer of octane) has an octane rating of 100 while heptane has a rating of 0. For example, an octane rating of 87 means the fuel is a mixture of 87% 2,2,4-trimethylpentane and 13% heptane, or any mixture of fuels or additives that have the same performance of 87/13. The gasoline fraction from crude oil possesses an octane rating of about 70, and so it cannot be used in a car directly which needs a minimum octane rating of 87.13
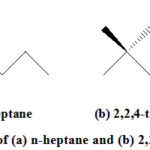 |
Figure 1: Structure of (a) n-heptane and (b) 2,2,4-trimethylpentane |
Tetraethyllead (TEL, Pb(C2H5)4)
In December 1921, Thomas Midgley, an American engineer and chemist while working for General Motors (GM), discovered that the addition of tetraethyllead [TEL, Pb(C2H5)4] to gasoline prevented knocking in internal combustion engines.4,14,15 Today, it is a well known fact, how Midgley came upon tetraethyllead as the additive that would reduce knocking after having combined gasoline with practically every substance, namely iodine to aniline.2
Synthesis of Tetraethyllead
The Kraus-Callis process forms the basis of the synthetic reaction used to obtain tetraethyllead.16 At first, molten sodium is combined with molten lead in a 1:1 ratio to form a reactive alloy which then reacts with ethyl chloride (CH3CH2C1) to form tetraethyllead.1
PbNa + 4CH3CH2C1 → 3Pb + 4NaC1 + Pb(C2H5)4 (1)
The product is then recovered by steam distillation.
The notable characteristic of TEL is the weakness of its four C–Pb bonds. At the temperatures obtained in the internal combustion engines, as Pb(C2H5)4 decomposes completely into lead, lead oxides and short-lived ethyl radicals. When as Pb(C2H5)4 burns completely in oxygen, the following reactions occur:
Pb(C2H5)4 + 13O2 → 8CO2 + 10H2O + Pb (2)
2Pb + O2 → 2PbO (3)
Pb and PbO remove the radical intermediates and thus break the radical chain reaction and make the intermediates of hydrocarbon oxidation (alkyl hydroperoxides) inactive, and thereby resist knocking. However, a significant limitation in the early use of TEL was the accumulation of PbO on the exhaust valves, spark plugs and combustion chamber so that it caused destruction of the engine. This deficiency was taken care of when in 1928 Earl Bartholomew changed the composition of the antiknock additive to include 1,2-dichloroethane and 1,2-dibromoethane, which acted as scavengers by converting the lead oxides to lead(II) chloride and lead(II) bromide, respectively, which being volatile under the operating engine temperature could be easily expelled from the engine and into the air.1 In this way lead was released into the environment from leaded fuels. After this hurdle was crossed, the production of leaded gasoline increased rapidly.
At this point, it will not be irrelevant to discuss a brief history of the synthesis of tetraethyllead. In 1853, Carl Lowig prepared tetraethyllead by the reaction of ethyl iodide with a sodium-lead alloy.2 However, ethyl iodide as the source of the ethyl group was not a good choice since it was very expensive. So, Midgley, and his coworkers used the relatively cheaper ethyl bromide to synthesize tetraethyllead. The reaction between the lead-sodium alloy and ethyl bromide was carried out in presence of pyridine,2 and the product was recovered by steam distillation. Pyridine (any other amine namely triethylamine can also be used) is believed to enhance the reactivity of the alkyl halide (bromide or iodide) used, by forming an intermediate addition compound as shown in Fig. 2.2
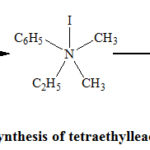 |
Figure 2: Role of an amine in the synthesis of tetraethyllead from ethyl iodide (Seyferth, 2003) |
The production of tetraethyllead from ethyl bromide was also not an ideal one since bromine was of limited supply and thus ethyl bromide was expensive. Around this time, Kraus and his associate Callis showed that the much cheaper ethyl chloride gave good results with 1:1 Na/Pb alloy and the yield was as high as 70 to 75%. With their work a practical process for the large scale production of tetraethyllead was established.2
Concern with Tetraethyllead: The Toxic Effects of Lead
The only concern with TEL was that it contained lead. It is a well known fact that lead is neurotoxic; harmful to humans and damages the central nervous system, kidney, liver and bones.17,18,19,20 Children are particularly vulnerable to the toxic effects of lead which can lead to a wide range of symptoms, from headaches and stomach pain to behavioural problems and anaemia. Lead also can affect a child’s developing brain.17,18,19,20 There is no known level of lead concentration in blood that is considered safe and as lead exposure increases, range and severity of its effects enhances.21 Midgley himself spent several months in Florida in 1923, recuperating from lead poisoning.22 Scientists and public health officials were alarmed and urged the government to look into the issue of health implications of using leaded gasoline in automobiles.
General Motors (GM) however made contracts with Standard Oil of New Jersey (now Exxon-Mobile), Standard Oil of Indiana (later Amoco and then BP) and Gulf Oil (owned by the Mellons) to manufacture TEL for them. Within a short time, several workers suffered from severe lead poisoning and died, but that fact was kept concealed at first. A year later, GM formed a joint venture with Standard and the Ethyl Corporation was formed. Within three months of its formation, again there was death of workers, this time 5 workers died and 35 were injured with symptoms such as tremors, hallucinations and severe palsies.23
Under pressure, from scientists, health officials and labour activists, the government organized a conference in Washington DC in May 1925.1 At one end there was Frank Howard, vice-president of the Ethyl Corporation, who opined that leaded gasoline was essential for development of motor fuels. On the other end was Dr. Alice Hamilton, the country’s leading authority on lead who stated clearly that lead was a chance not worth taking.
Dr. Henderson, another notable agitator, also clearly wrote his reservations against leaded gasoline in a letter that tetraethyllead cannot be introduced for general use until it is proved harmless.24
Throughout this period General Motors was of the opinion that ‘~the average street will probably be so free from lead that it will be impossible to detect it or its absorption”.1 The industries lobbied that great innovations involved some risk. To summarize it may be stated that an intensive industrial lobby was actually responsible to effectively stop any government regulation on lead in gasoline and leaded gasoline was approved for sale. For example, then, Ethyl demanded that the federal Public Health Service (PHS) hold hearings on TEL. But it was indeed surprising that the PHS was part of the Treasury Department, and Andrew Mellon – whose family had just signed a contract to distribute TEL through their Gulf Oil, was actually in charge of it.23
Phase-Out and Ban of Tetraethyllead
The mid 1960’s saw the beginning of the environmental movement and once again concerns were raised about the toxicity of lead additives in gasoline. Numerous reports were published, the foremost among them was that of Dr. Clair Patterson, which highlighted that automobiles were the main source of environmental lead pollution and voiced concerns about the continuous exposure to the large quantities of lead.25 Between 1953 and 1966, the concentrations of airborne lead in the United States averaged 1-3µg m-3 in urban areas.1 The Second National Health and Nutritional Examination Survey (NHANES II) held between 1976 and 1980 involving people of all ages in the United States revealed that 2.3~3.9 million children less than 5 years old had blood lead concentrations in excess of 250 µg/L.1 Continuous studies revealed that absorption of the automotive lead from the environment has become one of the most common public health hazards of modern civilization. The debate on toxic effects and economic benefits of lead intensified further which ultimately led to the start of “lead phasedown”. In the United States, the United States Environmental Protection Agency (EPA) ordered a scheduled phase out of lead content in gasoline under section 211 of the Clean Air Act. However, this was challenged by The Ethyl Corp in Federal court. The EPA’s regulation was initially dismissed,26 the EPA won the case on appeal, and the TEL phasedown finally started in 1976. Another point to be considered at this time was the advent of the use of catalytic converters in automobiles. A catalytic converter is a device that converts harmful chemicals like carbon monoxide, unburned hydrocarbons to carbon dioxide and water by reacting with oxygen, it also reduces oxides of nitrogen to nitrogen gas. Tetraethyl lead accumulated on these converters thus damaging them. As a result, cars with a catalytic converter preferred unleaded gasoline. Over the next decade, EPA sent out further regulations and in August 1984, it proposed a decrease in lead content to 0.1g per leaded gallon (gplg) by the start of 1986.3 The mandate of the EPA and the emergence and increasing use of other octane rating boosting substances brought about the end of widespread use of leaded gasoline. The final phaseout occurred still later when the the U.S. Clean Air Act banned the sale of leaded fuel for use in on-road vehicles from 1 January 1996.3
Tetraethyllead: a Social Perspective
It is interesting to consider the phaseout of tetraethyllead from the viewpoint of the Environmental Kuznets Curve.27,28,29 The Environmental Kuznets curve (EKC), demonstrates the relationship between economic progress and environmental deterioration with time. As an economy progresses,initially, various aspects of the environmental quality get worse, but with further economic growth the environment gradually gets cleaner. This is because that at the early stages of industrialization, with low income, people value material benefits more and tend to exploit the resources of nature and poison the environment. As income increases, people can become aware of the hazards of a polluted environment, and brings about regulatory measures to lower the environmental pollution. So, it may be stated that the ban on the use of leaded gasoline in U.S was also a result of the country reaching the further end of the Environmental Kuznets curve in the later part of the twentieth century.
By 2011, the United Nations declared that it had been able to successfully phaseout leaded gasoline worldwide. According to the United Nations Environmental Programme (UNEP), worldwide ban of leaded gasoline resulted in 2 million fewer early deaths, greater overall intelligence and 58 million lesser crimes.30 It was banned in India in March 2000.31 TheNairobi Convention of the UNEP (2017),5 presented the worldwide picture of leaded gasoline on March, 2017 as that shown in Fig.3.
 |
Figure 3: Worldwide use of leaded gasoline as in March 2017 (Nairobi Convention of the UNEP, 2017)5 |
In the United Sates after the TEL phaseout, the mean blood lead level of the population (aged 1 to 75 years) decreased from 12.8 μg/dL in 1976 to only 2.8 μg/dL in 1991.32 Various reports highlighted a strong connection between the rise in use of leaded petrol until the 1970s and a rise in violence. In the 1960s, U.S experienced a rapid increase in levels of violent crime. In the 1990s it started diminishing at a steady pace. Many researchers, the foremost being economist Jessica Reyes concluded that in the period between 1992 and 2002 the ‘phaseout’ of leaded gasoline in the U.S. caused an approximate decline of 56% in violent crime.33 It is intriguing to note that considering a 22-year time lag (reflecting the time for children damaged by the metal) the violent crime curve almost follows the lead exposure curve.33
Some neurologists have also put forward the belief that the tetraethyllead phaseout has been responsible for the rise in the average IQ levels by several points in the U.S since it has in general reduced the overall brain damage throughout the population, especially in children. It is indeed a studied fact that lead exposure has a negative effect on the intelligence quotient (IQ) of children.34
To summarise, the ascent and the decline of the use of leaded gasoline culminating in its ban stirs up the age old question; whether industrial and scientific progress for the convenience and comfort of mankind is worth the risk of a polluted environment. But in the case of leaded gasoline, the tragedy was much greater since all the people involved in its production and sale, knew it was toxic, but still went forward with it for financial benefits. Safer antiknock additives were available to the oil companies, the foremost being ethanol,35,36,37 but it was not considered as it could not be patented. The tale of tetraethyllead in gasoline provides caution about unregulated technology and how it can adversely affect the human race. At present, the use of leaded gasoline is prohibited in most countries.
Acknowlegement
The author acknowledges the Department of Chemistry, Hooghly Mohsin college, Hooghly, Chinsurah, West Bengal.
Conflicts of Interest
The author declares no conflict of interest.
References
- Nriagu, J. O. The rise and fall of leaded gasoline. The Sci. Total Env., 1990, 92, 13-28.
- Seyferth, D. The rise and fall of tetraethyllead. 2. Organometallics, 2003, 22(25), 5154-5178.
- Newell R. G.; Rogers. K. The market-based lead-phasedown. Resources for the Future (Discussion paper) 2003, 3-37.
- Midgley Jr., T. From the Periodic Table to production. Ind. Eng. Chem., 1937, 29 (2), 241-244.
- Nairobi Convention of United Nations Environment Programme, [online] web.unep.org/nairobiconvention/ (Accessed 5th March, 2018), 2017.
- Massenova, A.; Kalykberdiyev, M.; Ussenov, A.; Sass, A.; Kenzin, N.; Kanatbayev, E.; Baiken, A. Catalytic Technologies for the Production of Eco-friendly Gasolines and Reducing the Toxicity of Vehicle Exhaust Gases. Orient. J. Chem., 2019, 35(1), 351-357.
- Geetha, N. K.; Bridjesh, P.; Sekar, P. Influence of Ethanol as Gasoline Blend on Spark Ignition Engine. Orient. J. Chem., 2019, 35(5), 1491-1499.
- Sassykova, L. R. ; Aubakirov, Y. A.; Sendilvelan, S., Tashmukhambetova, Zh. Kh.; Faizullaeva, M. F.; Bhaskar, K.; Batyrbayeva, A. A.; Ryskaliyeva, R. G.; Tyussyupova, B. B.; Zhakupova, A. A.; Sarybayev, M. A. The Main Components of Vehicle Exhaust Gases and Their Effective Catalytic Neutralization. Orient. J. Chem., 2019, 35(1), 110-127.
- Ayoub, A. S.; Lucia, L. A. (Eds.). Introduction to renewable biomaterials: first principles and concepts, Wiley, New Jersey, 2017.
- Kovarik, B. Henry Ford, Charles Kettering and the fuel of the future. Automotive History Review, 1998, 32, 7-27.
- Hinton, D.D.; Olien, R. M. Oil in Texas: the gusher age, 1895-1945, University of Texas Press, Texas, 2002.
- MacCoull, N. Internal combustion engine, In Encyclopedia of Science & Technology, McGraw-Hill, New York, 1987, 305-317.
- Demirbas, A.; Balubaid, M. A.; Basahel, A. M.; Ahmad, W.; Sheikh, M. H. Octane rating of gasoline and octane booster additives. Petroleum Sci. Tech., 2015, 33(11), 1190-1197.
- Nickerson, S. P. Tetraethyllead: A product of American research. J. Chem. Education, 1954, 31, 560-571.
- Wilson, R. E. Perkin Medal: the medalist. Ind. Eng. Chem., 1937, 29(2), 239-241.
- Jewkes, J.; Sawers, D.; Stillerman, R. The Sources of Invention, 2nd ed., Palgrave Macmillan, UK, 1969.
- Duruibe, J. O.; Ogwuegbu, M. O. C.; Egwurugwu, J. N. Heavy metal pollution and human biotoxic effect. Int. J. Physical Sci., 2007, 2(5), 112-118.
- Landrigan, P. J.; Boffetta, P.; Apostoli, P. The reproductive toxicity and carcinogenicity of lead: a critical review. American J. Ind. Medicine, 2000, 38, 231-243.
- Navas-Acien, A.; Guallar, E.; Silbergeld, E. K. S.; Rothenberg, J. Lead exposure and cardiovascular disease-a systematic review. Env. Health Perspectives, 2007, 115(3), 472–482.
- Wani, A. L.; Ara, A.; Usmani, J. A. Lead Toxicity: a review. Interdisciplinary Toxicology, 2015, 8(2), 55–64.
- World Health Organization, Media Centre, Fact Sheet, updated August, [online] www.who.int/mediacentre/factsheets/ (Accessed 5th March, 2018), 2017.
- Jack, A. They Laughed at Galileo: How the Great Inventors Proved Their Critics Wrong. Skyhorse Publishing, New York, USA, 2015.
- Ganzel, B. Oil Vs. Ethanol. https://livinghistoryfarm.org/farminginthe50s/crops_05.html (Accessed 5th March, 2018), 2007.
- Rosner, D.; Markowitz. G. A ‘gift of God’?: The public health controversy over leaded gasoline during the 1920s. American J. Public Health, 1985, 75(4), 344-352.
- Patterson, C.C. Contaminated and natural lead environments of man. Archives Env. Health: an Int. J., 1965, 11(3), 344-360.
- Kovarik, W. Ethyl-leaded Gasoline: How a Classic Occupational Disease Became an International Public Health Disaster. Int. J. Occupational Env. Health, 2005, 11(4), 384-397.
- Dasgupta, S.; Laplante, B.; Wang, H.; Wheeler, D. Confronting the Environmental Kuznets Curve. The J. Economic Perspectives, 2002, 16(1); 147-168.
- Dinda, S. Environmental Kuznets Curve Hypothesis: a survey. Ecol. Economics, 2004, 49, 431– 455.
- Stern, D. I. The Environmental Kuznets Curve. Int. Society for Ecol. Economics, Internet Encyclopaedia Ecol. Economics, [online] isecoeco.org/pdf/stern.pdf, 2003.
- Tsai, P. L.; Hatfield, T. H. Global benefits from the phaseout of leaded fuel. J. Env. Health, 2011, 74(5), 8–14.
- Venkatesh, T. A surprising source of lead poisoning: India’s Idols. The Wall Street Journal, Jun 4, 2:11 pm, 2015.
- Pirkle, J. L., Brody, D. J., Gunter, E. W.; Kramer, R. A.; Paschal, D. C.; Flegal, K. M.; Matte. T. D. The decline in blood lead levels in the United States. The National Health and Nutrition Examination Surveys (NHANES). The J. American Medical Association, 1994, 272(4), 284-291.
- Reyes, J. W. Environmental policy as social policy? The impact of childhood lead exposure on crime. The B.E. J. Economic Analysis Policy, 2007, 7(1), 1-51.
- Lanphear, B. P.; Hornung, R.; Khoury, J.; Yolton, K.; Baghurst, P.; Bellinger, D. C.; Canfield, R. L.; Dietrich, K. N.; Bornschein, R.; Greene, T.; Rothenberg, S. J.; Needleman, L.; Schnaas, H. L.; Wasserman, G.; Graziano, J.; Roberts, R. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Env. Health Perspectives, 2005, 113(7), 894–899.
- Kovarik, W. Ethyl: The 1920s environmental conflict over leaded gasoline and alternative fuels. Proceedings of the American Society of Environmental History, Annual Conference, Providence, R.I., USA, 2003.
- Lester, J. P. (Ed.). Environmental Politics and Policy: theories and evidence. John Mckormick, London, 2018.
- Porter, J. C.; Wiebe, R. Alcohol as an antiknock agent in automotive engines. Ind. Eng. Chem. Res., 1952, 44(5), 1098-1104.

This work is licensed under a Creative Commons Attribution 4.0 International License.










