Metal Complexes of Hybrid Oxygen- Arsenic Ligands-VII IR, Reflectance and EPR Spectral Studies of Oxo-manganese (II)-Arsine Complexes Using Ligands from o-R2AsC6H4CO2H
S.S. Parmar1, A. Aggarwal 2, M. L. Sehgal3*, Midas Tsai2 and S. Mittal4
1Department of Chemistry, G. N. D. U., Amritsar, 143005, India.
2Department of Natural Sciences, LaGuardia Community College of the City University of New York, 31-10 Thomson Avenue, Long Island City, New York, NY 11101, United States
3Department of Chemistry, D.A.V. College, Jalandhar, 144008, India
4School of Chemistry and Biochemistry, Thapar Institute of Engineering & Technology, Patiala,147004, India
Corresponding Author E-mail: manoharsehgal@hotmail.com
DOI : http://dx.doi.org/10.13005/ojc/360106
Article Received on : 09-Dec-2019
Article Accepted on : 10-Jan-2020
Article Published : 14 Jan 2020
This paper describes four oxo-manganese(II)- mono-tertiary arsine complexes: [Mn2O{(o-Ph2AsC6H4CO2)2(H2O)5}.(H2O)](structure:4),[Mn2O(o-Ph2AsC6H4CO2)2(H2O)4], and two isomeric compounds of [Mn2O{(o-(p-tolyl)2AsC6H4CO2)2}.(H2O)4] (structure 5) by reacting Mn(O2CMe)2. nH2O, (where “n” represents the number of water molecules, n=0 and n= 4) with the ligands o-R2As C6H4 CO2H having only the aryl substituents (R=Ph, p-tolyl). This work extends the argument further that deoxygenated anhydrous conditions are not a prerequisite to stabilize As(III)-Mn(II) bond with the hybrid (As-O) chelates. The prepared complexes were characterized by IR, reflectance and EPR spectral studies and were confirmed by elemental analysis and thermogravimetric studies for their structures.
KEYWORDS:Mono-tertiary arsine; Molar Conductance; Magnetic Moment; Spin-lattice Relaxation; Thermogravimetric Analysis
Download this article as:| Copy the following to cite this article: Parmar S. S, Aggarwal A, Sehgal M. L, Tsai M, Mittal S. Metal Complexes of Hybrid Oxygen- Arsenic Ligands-VII IR, Reflectance and EPR Spectral Studies of Oxo-manganese (II)-Arsine Complexes Using Ligands from o-R2AsC6H4CO2H. Orient J Chem 2020;36(1). |
| Copy the following to cite this URL: Parmar S. S, Aggarwal A, Sehgal M. L, Tsai M, Mittal S. Metal Complexes of Hybrid Oxygen- Arsenic Ligands-VII IR, Reflectance and EPR Spectral Studies of Oxo-manganese (II)-Arsine Complexes Using Ligands from o-R2AsC6H4CO2H. Orient J Chem 2020;36(1). Available from: https://bit.ly/35RgNLx |
Introduction
Transition metal complexes with organic ligands are of great interest of researchers since the discovery of first such complex compound of Ni(II) with azabenzene ligand in early 1960s.1 Since then a wide variety of cyclometallated complex compounds of transition and post transition metal ions with ligands containing nitrogen, phosphorous, arsenic, Oxygen, sulfur etc. as donor atoms has been investigated thoroughly.2 The complexes of manganese (II) with arsines ligands are not very common as the arsines are very much susceptible to oxidation even in the presence of traces of moisture when reacted with Mn (II) salts like manganese (II) halides react with AsPh3 to form complexes like [Mn(OAsPh3)2X2]3,4 where AsPh3 would oxidize to arsine oxide.
Six manganese (II)-mono-tertiary arsine complexes had been reported in literature.5,6 Chiswell et al.5 were able to stabilize two manganese (II)-arsine complexes [Mn(As-N)X2] (where, X= Br or ClO4) with arsenic-nitrogen chelating agent-o-dimethylarsinoaniline even in the presence of both oxygen and water the latter was removed from the mixture azeotropically. Parmar et al.6 reported four manganese (II)- mono-tertiary arsine complexes [Mn{(o-Me2AsC6H4CO2)Cl}.½H2O] and [Mn{(o-R2 AsC6H4CO2)2(H2O)2}. nH2O] (R=Ph, p-tolyl, n=0; and R=Et, n=1) formed by reacting MnCl2.4H2O with o-R2AsC6H4CO2Na (R= Me, Et, Ph, p-tolyl) in 1:2 molar ratio in 95% EtOH. All these four complexes were also isolated in the presence of both moisture and oxygen.
We previously6 were able to prove that McAuliffe’s emphasis3,4,7,8 on the use of strictly non arial anhydrous reaction medium for stabilizing As(III)-Mn(II) bond would hold good only for soft ligands and not for (As-O) hybrid ligands. We, now, extend this argument further by using both the anhydrous Mn(O2CMe)2 and hydrated Mn(O2CMe)2.4H2O in 95% Et OH as well as ethanol with o– R2AsC6H4CO2H instead of o-R2AsC6H4CO2Na. Our emphasis would, also include studying the effect of counter anion by replacing chloride ion by acetate ion.
Experimental Details
Details of spectral and other measurements, preparation of [(Structure 1, Fig. 1); M=H, Na] and estimation of arsenic (III) were published elsewhere9-13 A brief experimental details of spectral and magnetic moment measurements are shown below in 2.2 and 2.3. Analytical grade Mn(O2CMe)2. 4H2O was used for the preparation of the complexes. Anhydrous Mn(O2CMe)2 was prepared by heating Mn(O2CMe)2. 4H2O in a dry gun for 3-4 h and the resulting mass was cooled in vacuo. The vacuo refers to distillation using a rotary evaporator attached to an efficient vacuum pump. The process was repeated till its IR shows no bands characteristic of water.
Preparation of Complexes of Manganese (II)
Preparation of [Mn2O{(o-Ph2AsC6H4CO2)2(H2O)5}.(H2O)] (Structure 4)
Addition of a hot ethanolic solution (18-20 cm3) of [(Structure 1, Fig. 1); R=Ph & M=H] (8 mmol) and anhydrous Mn(O2CMe)2 (0.692 g, 4 mmol) in ethanol (30-35 cm3) gave a white solid. The reaction mass was further refluxed for 12 h. The solid was filtered and washed with EtOH, Et2O and dried in vacuo. Yield: 50-55%.
Preparation of [Mn2O{(o-Ph2AsC6H4CO2)2(H2O)4}] (Structure 5)
The solution of [(Structure 1, Fig. 1); R=Ph & M=H)] (8 mmol) and Mn(O2CMe)2.4H2O (0.98 g, 4 mmol) in 95% Et OH (40 cm3) was refluxed for 2h and resulted into a clear solution. The addition of Et2O to this clear solution gave a white solid which was filtered, washed with Et2O and dried in vacuo. Yield: 50-60%.
Preparation of [Mn2O{(o-(p-tolyl)2AsC6H4CO2)2(H2O)4}] (Structure 5)
The reaction mass obtained by the dropwise addition of Mn(O2CMe)2 (0.692 g, 4 mmol) in 95% Et OH (25-35 cm3) to a solution of [(Structure 1, Fig. 1); R=p-Tolyl & M=H] (8 mmol) in the same solvent (18-20 cm3) and was refluxed for 12 h. The reaction mixture was cooled to 0-5 oC. The solid, thus, obtained was filtered, washed with 95% Et OH, Et2O and dried in vacuo. Yield: 40-50%.
Spectroscopic Measurements
Infrared Spectra
The infrared spectra of the prepared complexes were recorded PYE UNICAM Sp3-300 IR Spectrophotometer in the range 4000-200 cm-1 on KBr pellets.
Electronic Absorption Spectra
The electronic absorption spectra of the prepared complexes were recorded using VSU-2P (DDR) spectrophotometer in the range 10000-30000cm-1 in solid state using magnesium oxide as the standard reflector.
Electron Paramagnetic Resonance (EPR) Spectra
The powder pattern EPR of the complexes at room temperature were recorded at R.S.I.C., I.I.T., Madras using Varian Spectrophotometer having a constant microwave frequency of 9.3 GHz( X-band;0-10000G). The g values were calculated by using the formula hn= gbH where H is the magnetic field in gauss measured at the point where the peak appears.
Conductance Measurements
The molar conductance of millimolar solutions of the complexes in PhNO2 or CH2Cl2 were measured on Toshniwal Conductivity Bridge Type CLOI/O2A using conventional dip type platinum electrode.
Thermogravimetric Analysis
The thermogravimetric analyses of the complexes were carried out on a manual thermo-balance (FCI) at the heating rate of 100 C/min and % loss in weight was plotted against temperature.
Magnetic Susceptibility Measurements
Magnetic susceptibilities of the powdered samples of the complexes were measured at room temperature using Gouy’s method. Diamagnetic corrections for the ligand anions (o-R2AsC6H4CO2) – were calculated using Pascal’s Law constants (R=Me, -113.6 x 10-6/mole; Et, -137.3 x 10-6/mole; C6H11, -210.4 x 10-6/mole; Ph, -188.1 x 10-6/mole and p-tolyl, -205.9 x 10-6/mole).
Results and Discussion
Elemental [C, H, N, Mn] (Table: 1) and thermogravimetric data (Table:2) authenticated the correctness of formulae of the oxomanganese (II)-arsine complexes which behaved as nonelectrolytes in PhNO2 or CH2Cl2. Their molecular weights could not be determined as the complexes got separated as solids under cryoscopy conditions.
Table 1: Elemental Data of Manganese (II) Complexes.
| Formula of Complex Compound ( M. Pt 0C) | Color* of the Complex Compound | Found (Calcd)% | |||
| C | H | As(III) | Mn(II) | ||
| [Mn2O{(o-Ph2AsC6H4CO2)2(H2O)4}] (175 oC) | Dirty White | 50.2(50.9) | 3.8(4.0) | 16.3(16.6) | 12.8(12.3) |
| [Mn2O{(o-Ph2AsC6H4CO2)2(H2O)5}H2O] (212 oC) | Light Pink | 47.8(48.9) | 4.1(4.3) | 15.8(16.1) | 12.1(11.8) |
| [Mn2O{(o-(p-tolyl)2AsC6H4CO2)2 (H2O)4}] ( ˃300 oC) | Light Pink | 52.2(52.9) | 4.7(4.6) | 16.0(15.8) | 12.0(11.6) |
| [Mn2O{(o-(p-tolyl)2AsC6H4CO2)2 (H2O)4 }] ( ˃300 oC) | Light Pink | 52.2(52.9) | 4.2(4.6) | 15.2(15.8) | 11.9(11.6) |
* A white compound may change to dirty white or light pink on drying.
Table 2: Thermal Analysis and Molar Conductance Data of Manganese (II) Complexes.
| Formula of Complex Compound | Thermal Analysis | Λ (S cm2mol-1) in PhNO2(CH2Cl2) | |
| Temp. Range 0C | Loss%
Found (Calcd.) |
||
| [Mn2O(o-Ph2AsC6H4CO2)2(H2O)4] | 100-240 | 8.0(8.0; 4H2O) | — |
| [Mn2O{(o-Ph2AsC6H4CO2)2 (H2O)5}.H2O] | 80-120120-200 | 1.75(1.91; H2O)9.25(9.64; 5H2O) | 0.2 |
| [Mn2O{(o-(p-tolyl)2AsC6H4CO2)2(H2O)4}] | 100-160160-215 | 3.75(3.75; 2H2O)3.75(3.75; 2H2O) | — |
| [Mn2O{(o-(p-tolyl)2AsC6H4CO2)2(H2O)4}] | — | — | — |
Presence of Bent Mn(II)-O-Mn(II) System in the Complexes
Contrary to our previous study6 of reacting same ligands with [MnCl2.4H2O], a strong new band always appeared in 560-610 cm-1 region for all the four synthesized complexes (Table: 3) that indicates the presence of a bent Mn(II)-O-Mn(II) system in the complexes.
Table 3: IR Spectral (cm-1) and Magnetic Moments Data of Manganese (II) Complexes.
| Formula of Complex Compund | νOH, [δHOH] | ν(asym CO2) | ν(sym CO2) | ν(Mn-O-Mn) | Structure |
| [Mn2O(o-Ph2AsC6H4CO2)2(H2O)4] | 3610 w, 3430 brm, [1610 sh] | 1595 vs | 1390 vs | 560 s | 5 |
| [Mn2O(o-Ph2AsC6H4CO2)2(H2O)5} .H2O] | 3430 brm, [1615 sh] | 1602 vs | 1405 vs | 610s | 4 |
| [Mn2O{(o-(p-tolyl)2AsC6H4CO2)2 (H2O)4 }] | 3360 brm, [1620 sh] | 1589 vs | 1378 vs | 590s | 5 |
| [Mn2O{(o-(p-tolyl)2AsC6H4CO2)2 (H2O)4}] | 3400 brm, [1630 sh] | 1580 s | 1395 s | 570s | 5 |
Brm = broad medium intensity; s = strong; vs = very strong; w = weak; sh = shoulder
Bonding Mode of Carboxylate ion in Oxomanganese (II)-Arsine Complexes
The IR spectra of the complexes resembled with those of [(Structure 1, Fig. 1)17; M=H] in 700-900 cm-1 region which possessed strong ρC-H14 and δC-C14 bands at 740 and 690 cm-1 respectively and were always accompanied by a weak δOCO14 band at 835 cm-1. Application of direction shift (d.s.) criterion9,15,16 to the IR data (Table: 3) of the four complexes in the carboxylate region would suggest (structure 2; Fig:1)17 involving the simultaneous coordination of As(III) and carboxylato oxygen to Mn(II) to rule out (structure 3, Fig:1)16. The widely different values of the marked bands in the two compounds with molecular formula [Mn2O{(o-(p-tolyl)2AsC6H4CO2)2(H2O)4}] is confirmation of their isomeric forms. The presence of oxo-bridged H2O molecule in three of the four studied complexes, though difficult to ascertain only with vibrational spectral studies, yet the presence of broad band in the range of 930-50 cm-1 points towards the presence of water in their structures.
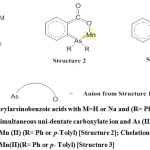 |
Figure 1: o-Diarylarsinobenzoic acids with M=H or Na and (R= Ph or p– Tolyl) |
![Figure 2: Top: Structure of Oxomanganese(II)-arsine complex, [Mn2O{(o-R2AsC6H4CO2)2(H2O)n}. n'(H2O)] (R=Ph, n=5, n' =1) (left) and its ball and stick model (right)](http://www.orientjchem.org/wp-content/uploads/2020/01/Vol36No1_ment_ssp_fig2-150x150.jpg) |
Figure 2: Top: Structure of Oxomanganese(II)-arsine complex, [Mn2O{(o-R2AsC6H4CO2)2(H2O)n}. n‘(H2O)] (R=Ph, n=5, n’ =1) (left) and its ball and stick model (right) |
Presence of Water in Complexes
Surprisingly, the complexes obtained under anhydrous conditions shown in Reaction 2.1.1 possessed either the same number of water molecules or even more than the corresponding complexes isolated from the hydrated conditions shown in Reactions 2.1.2 and 2.1.3. This could only be explained based on the absorption of moisture during the manipulations of Reaction 2.1.1. The presence of water molecules in the prepared complexes was indicated by the appearance of a strong band νOH (H2O)19 at ≈ 3350 – 3400 cm-1 and δHOH(sh)20 at ≈ 1600 – 1630 cm-1. Rocking mode of coordinated water ρ(H2O)21 found at ≈ 800 – 900 cm-1 was obscured by δOCO14 / νAs=O10 bands at 835 – 870 cm-1 region. The water molecules present in the complexes lost up to 100 0C (T.G.A. data; Table: 2) were regarded as lattice or loosely coordinated while the remaining water molecules were strongly coordinated to the metal ion (Table: 2).22 In the complex compound [Mn2O(o-Ph2AsC6H4CO2)2(H2O)5}H2O], one water molecule that is present outside the bracket (}) is lost below 120 oC indicates that it is a either a loosely bound lattice water molecule. On the other hand, the stoichiometric loss of other five water molecules (% loss = 1.75 : 9.25 = 1 : 5) in this complex beyond 120 oC points towards the presence strongly bound coordinated bond with Mn(II) metal ion in the complex (Table: 2).20
Magnetic Moments of Complexes
The formation of the bent Mn(II)-O-Mn(II) system was expected to result only a marginal lowering of magnetic moment values in contrast to the linear bridging which would cause a drastic lowering. Probably, no bent Mn(II)-O-Mn(II) systems were known whereas in the analogous isoelectronic Fe(III)-O-Fe(III) systems, the formation of σ and π bonds was invoked to explain the spin exchange coupling between M-3d and O-2p orbitals.23-26 The experimental μeff values of these oxo-manganese (II) complexes would lie in the range of 5.12 – 5.90 B.M. and, thus, indicated the presence of high spin Mn(II) with 6A1g ground having no contribution from TIP (Temperature Independent Paramagnetism).
Reflectance Spectra of Complexes
The room temperature magnetic moment values of the four Mn (II) complexes suggest that they have octahedral stereochemistry. This is further, corroborated by their reflectance electronic spectra which showed very week bands27 due to their doubly forbidden nature as neither they obey the multiplicity rule nor the symmetry (Laporte) rule. With five unpaired electrons, these Mn (II) complexes have six multiplicity with 6S ground state and the symmetry symbol 6A. The ground term is represented by 6A1g where ‘g’ stands for gerade in octahedral stereochemistry. The spectroscopic state immediately higher to 6S state is 4G which splits up into 4T1g, ,4T2g , 4A1g , 4Eg in an octahedral field. Three spectral bands arising from 6A to 4G are assigned to as 6A1g → 4T1g (4G), 6A1g→4T2g (4G) and 6A1 → 4A1g = 4Eg (4G) as the last two terms do not differ very largely in their energies. The fourth band occurs by the transition between the ground 6A1g term to 4T2g obtained by the splitting of 4F which is immediately higher in energy to 4G (Table:4).
Table 4: Electronic Spectral (cm-1) and Tentative Structures of Manganese (II) Complexes.
| Complex | →4T1g | →4T2g | →4A1g. 4Eg | →4T2g | μeff (B.M.) |
| [Mn2O(o-Ph2AsC6H4CO2)2(H2O)4] | 18182, 17000 | 21050 | 24390 | 25600 | 5.12 |
| [Mn2O(o-Ph2AsC6H4CO2)2(H2O)5} .H2O] | 18182, 17000 | 21252 | 24352 | 25640 | 5.74 |
| [Mn2O{(o-(p-tolyl)2AsC6H4CO2)2 (H2O)4}] | 16625 | — | — | 25640 | 5.53 |
| [Mn2O{(o-(p-tolyl)2AsC6H4CO2)2 (H2O)4}] | 16625 | 20000 | 22471 | 25640 | 5.90 |
EPR Spectra of Complexes
Only [Mn2O{(o-Ph2AsC6H4CO2)2(H2O)4}] complex gave EPR signal at room temperature in the form of a broad peak with g ≈ 2.0 which indicated its nearly axial symmetry having small distortion from octahedral stereochemistry with 6A1g ground term.28 It was quite likely that the lines of the system had their EPR resonance broadened beyond distinction due to spin-lattice relaxation. Further, this might also, be due to magnetic exchange between manganese (II) ions in the oxo- complexes, i.e., coupling between Mn-3d and O-2p orbitals23-26 as had already been predicted based on the magnetic data.
Structures of the Four Oxomanganese (II)-Arsine Complexes
The IR spectra of the complexes indicated the presence of bent Mn(II)-O-Mn(II) unit. The electronic absorption, EPR spectra and magnetic data complemented one another to confirm their almost octahedral stereochemistry around Mn(II). The thermal data showed the presence of four coordinated water molecules in three complexes: [MnO2{(o-R2As C6H4CO2)2(H2O)4}], {R=Ph, p-tolyl (two isomers)} and five coordinated water molecules and one loosely bound lattice water molecule in the fourth complex: [MnO2{(o-Ph2AsC6H4CO2)2(H2O)5}.H2O]. Thus, all the techniques used in this study corroborated well to assign them the tentative structures 4, 5; Fig: 1 (Table:3).
Conclusions
Though we obtained oxomanganese(II)-mono-tertiary arsine complexes and not the manganese(II)-mono-tertiary complexes, yet As(III) was not oxidized to As(V) oxide contrary to McAuliffe’s emphasis3,4,7,8 on the use of strictly deoxygenated anhydrous reaction medium for stabilizing As(III)-Mn(II) bond which highlighted the importance of inductive effect in restricting the oxidation of As(III) to As(V) in ligands with aryl substituents (R=Ph or p-tolyl). It, also, justified the effect of counter anion as chloride would affect neither As(III) nor Mn(II) while the acetate ion would change manganese (II) to oxo-manganese (II) without oxidizing As(III) to As(V) oxide.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgement
We are greatly thankful to Professor Surjeet Singh of R.S.I.C., I.I.T., Madras for providing E.P.R. instrumental facilities and to Professor S. Subramanian of R.S.I.C., I.I.T., Madras for fruitful discussion of the E.P.R. data.
References
- Kleiman, J. P., Dubeck, M., The Preparation of Cyclopentadienyl [o-(Phenylazo) Phenyl] Nickel., Am. Chem. Soc. 1963, 85(10), 1544-1545.
- Mohr, F., Privér, S. H., Bhargava, S. K., Bennett, M. A., Ortho-metallated transition metal complexes derived from tertiary phosphine and arsine ligands., Chem. Rev., 2006, 250(15-16), 1851-1888.
- Casey, S., Levason, W., McAuliffe, C. A., Co-ordination chemistry of manganese. Part –III. The reaction of manganese (II) halides with tertiary phosphine and arsine ligands to produce ligand oxide complexes., Chem. Soc. Dalton Trans., 1974, 886-889.
- Levason, W., McAuliffe, C.A., The coordination chemistry of manganese—IV. The reaction of manganese(III) chloride with group VB ligands[1]., Inog. Nuc. Chem., 1975, 37(1), 340-342.
- Chiswell, B., Plowman, R. A., K. Verrall., Metal Complexes of Some Hybrid Ligands Containing Tertiary Arsine and Primary Amine Donor Groups, I. Compounds of Mn, Fe and CO. Metal complexes of some hybrid bidentate ligands containing, Chim. Acta., 1971, 5, 579-589.
- Parmar, S. S., Bharj, H. K., Sehgal, M. L., Metal Complexes of Hybrid Oxygen- Arsenic Ligands-VI. Stabilization of Manganese-Arsine Complexes using Ligands from o-R2AsC6H4CO2, Polyhedron, 1987, 6(8), 1699-1701.
- Jones, M. H., Levason, W., McAuliffe. C. A., Parrott, M. J., The co-ordination chemistry of manganese. Part- V. The preparation and spectroscopic characterization of some manganese (II) complexes of arsenic and antimony donor ligands., Chem. Soc. Dalton Trans., 1976, 7(6), 1642-1645.
- C.A., Studies on tetrahydrofuran solutions of manganese(II) dihalides and tertiary phosphines and their reactions with dioxygen. A reply to a paper by green, mingos and coworkers., J. Organomet. Chem., 1982, 228, 255-263.
- Parmar, S. S., Kaur, H., Metal complexes of hybrid oxygen- arsenic ligands-Part I. Complexes of o-dialkylarsinobenzoic acids with chromium (III)., Met. Chem., 1982, 7, 79-85.
- Parmar, S. S., Bharaj, H. K., Sehgal, M. L., Metal complexes of hybrid arsenic ligands- V. A study of products from o-R2AsC6H4CO2M and CrO3 (M=H/trans CrCl2(OH2)4 2H2O (M=Na) in acetone., Trans. Met. Chem., 1986, 11, 283-286.
- Vogel, A.I. “A text book of qualitative Inorganic analysis, ELBS and Longman, London,” 7th Edition, 2010.
- Bain, G. A., Berry, J. F., Diamagnetic corrections and Pascal’s constants., Chem. Educ., 2008, 85(4), 532.
- Saighal, M. L., “D. Thesis, “Studies on metal ion complexes of arsenic-oxygen hybrid chelating agents”, G. N.D. University Amritsar. (India) 1986.
- Taylor, M.D., Carter, C.P., Wynter, C. I., The infra-red spectra and structure of the rare-earth benzoates., Inorg. Nucl. Chem., 1968, 30(6), 1503-1511.
- Parmar, S., Basra, T. S., Sandhu, S. S., Stabilization of copper(II)tertiary-phosphine or arsine complexes. Part- I. Complexes of copper (II) with hybrid oxygen-arsenic ligands from o and p-(Diphenylarsino) Benzoic Acids., J. Chem. Soc. Dalton Trans., 1982, 1939-1943.
- Manhas, B.S., Trikha, A. K., Relationship between the Direction of Shift in the carbon- oxygen stretching frequencies of carboxylate complexes and the type of coordination. Indian Chem. Soc. 1982, 59, 315 -319.
- S. S., Kaur, H. Metal complexes of hybrid oxygen-arsenic ligands—III: Complexes of 2-tertiary arsinobenzoic acids with zinc(II), cadmium(II) and mercury(II). Discovery of a novel chelating system, Polyhedron, 1982, 1 (9, 10), 667-671.
- Parmar, S. S., Bathla, H. K., Indian J. Chem., 1985, 24 A, 1035. Cross Reference: Met. Chem., 1986, 11, 283-286.
- Adams, D. M. Metal Ligands and related vibrations., Edward Arnold, London. 1967, p.238
- Infrared and Raman Spectra of Inorganic and Coordination Compounds Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, by Nakamoto., Wiley & Sons, Incorporated, John, January 2009.
- Fujita, J., Nakamoto, K., Kobajashi, M., Infrared spectra of metallic complexes. II. The absorption bands of coordinated water in aquo complexes. , Am. Chem. Soc. 1956, 78 (16), 3963-3965.
- Kavitha, N., Anantha Lakshmi, P.V., Synthesis, characterization and thermogravimetric analysis of Co(II), Ni(II), Cu(II) and Zn(II) complexes supported by ONNO tetradentate Schiff base ligand derived from hydrazino benzoxazine., Saudi Chem. Soc., 2017, 21, S457-S466.
- Ondrejkovicova, I., Lis, T., Mrozinski, J., Vancova, V., Melkin, M., Synthesis, structure, spectra and magnetic properties of the unsymmetrical oxo-bridged complex [Fe2O(OAsPh3)4Cl3]2[Fe2OCl6].2CH3CN. Polyhedron, 1998, 18, 3121-3130.
- Cauchy, T., Ruiz, E., Alvarez, S., Exchange coupling interactions in a Fe6 complex: A theoretical study using density functional theory. Physica B Condens. Matter., 2006, 384(1), 116-119.
- Davies, J. E., Gatehouse, B. M., The crystal and molecular structure of μ-oxo-bis[bis-(N–p– chlorophenylsalicylaldiminato)iron(III)]., Acta Cryst., 1973, B29(12), 2651-2658.
- Mabbs, F. E., McLachlan, V. N., McFadden, D., McPhail, A. T. Magnetic properties and crystal and molecular structure of µ-oxo-bis[bis-(2-methyl-8- hydroxyquinolinatoiron (III) –chloroform, Chem. Soc. Dalton Trans., 1973, 2016-2021.
- Emara A. A. A., Ali, A. M,, El-Asmy, A. F., Ragab,El-S. M., Investigation of the oxygen affinity of manganese(II), cobalt(II) and nickel(II) complexes with some tetradentate Schiff bases., Saudi Chem. Soc., 2014, 18(6), 762- 773.
- Sasi, S., Sithambaresan, M., Kurup, M. R. P., Fun, H.-K., Syntheses, EPR spectral studies and crystal structures of manganese (II) complexes of neutral N,N donor bidentate Schiff bases and azide/thiocyanate as coligand, Polyhedron, 2010, 29(13), 2643-2650.

This work is licensed under a Creative Commons Attribution 4.0 International License.









