Evaluation of Fatty Acid Composition and Antimicrobial Activity of Eight Medicinal Plants from Kashmir
Zubair Rehman Nengroo1, Abdul Rauf1*, Mohammad Danish2, Muzammil Sharief Dar3
1Section of oils and fats, Department of Chemistry, Aligarh Muslim University, Aligarh 202002, Uttar Pradesh, India
2Department of Botany, Aligarh Muslim University, Aligarh 202002, Uttar Pradesh, India.
3Department of Microbiology, Aligarh Muslim University, Aligarh 202002, Uttar Pradesh, India.
Corresponding Author E-mail: abduloafchem@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/360107
Article Received on : 30 Nov 2019
Article Accepted on : 15 Jan 2020
Article Published : 04 Feb 2020
The seeds of Abrus precatorius Linn., Amaranthus virdis Linn., Bunium persicum Boiss., Dioscorea deltoidea Wall. Ex Griseb., Malva neglecta Wall., Podophylum hexandrum Royle., Robina pseudoacacia Linn. and Teraxacum officinale Weber were used for fatty acid composition and antimicrobial activity. By GC-MS analysis, the petroleum extracts of seed were rich in oleic acid with 60.2%, 58.9% and 57.5% for A. precatorius, A. virdis and P. hexandrum respectively. Linoleic acid was dominant in M. neglecta 61.8%, T. officinale 59.0% and R. pseudoacacia 46.3% and petroselinic acid in B. persicum 64.0%. The defatted seed extracts showed strong inhibition zones in (mm) against fungus Aspergillus niger (14.75-16.45 with nystatin 20.36), Aspergillus fumigates (19.48-19.74 with nystatin 25.56) and Penicellium marneffei (21.95-23.93 with nystatin 28.50) and strongest MIC values (µg/ml) of 150, 250 and 500 against bacteria Esxherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa. This study exhibited beneficial properties of these plants in food and pharmaceutical industries.
KEYWORDS:Antifungal; Antibacterial; Gas Chromatography-Mass Spectrometry Oleic Acid; Linoleic Acid; Soxhlet Extraction.
Download this article as:| Copy the following to cite this article: Nengroo Z. R, Rauf A, Danish M, Dar M. S. Evaluation of Fatty Acid Composition and Antimicrobial Activity of Eight Medicinal Plants from Kashmir. Orient J Chem 2020;36(1). |
| Copy the following to cite this URL: Nengroo Z. R, Rauf A, Danish M, Dar M. S. Evaluation of Fatty Acid Composition and Antimicrobial Activity of Eight Medicinal Plants from Kashmir. Orient J Chem 2020;36(1). Available from: https://bit.ly/393siBy |
Introduction
Abrus precatorius Linn., Amaranthus virdis Linn., Bunium persicum Boiss., Dioscorea deltoidea Wall. Ex Griseb., Malva neglecta Wall., Podophylum hexandrum Royle., Robina pseudoacacia Linn. and Teraxacum officinale Weber are indigenous plants in Kashmir with low care cost. These plants are found in much abundance with numerous nutritional1,2,3 and therapeutic4,5,6,7,8,9,10 properties. Moreover the seeds of these plants could be an important source of fatty acids and antimicrobial agents.The traditional uses of these plants are given in Table 1. Lipids are the main constituents of food. They are most dominantly found in seeds of plants. The high degree of unsaturated fatty acids present in lipid helps to overcome various cardiovascular diseases26. Fatty acids mostly occur in natural fats and plant oils and they contain important nutritional and metabolic substances in living organisms27. The unsaturated fatty acids linoleic acid and Linolenic acid are very important for human health28, while as oleic and linoleic acids can act as vehicles for transfer of other active ingredients, dissolved or dispersed in oil-water type emulsions. The antioxidants could be blended with unsaturated fatty acids to increase their activity e.g. the blends of oleic acid with tocopherol have better protective effect than α-tocopherol itself. Moreover over replacing saturated fats with unsaturated fats could decrease total and low density (LDL) cholesterol in foods. Excessive use of Antibiotics and synthetic drugs leads to serious health issues both in plants, humans and indirectly to whole food chain which leads to serious problems29. There is recent trend in search for the discovery of new drugs which are less expensive, eco-friendly, biodegradable, safer and natural30,31. Plants are a source of phytochemicals which can themselves used as pesticides which are biodegradable, less toxic to plants and animals32. Therefore keeping in consideration of their much abundance and tremendous biologically properties of A. precatorius, A. virdis, B. persicum, D. deltoidea, M. neglecta, P. hexandrum, R. pseudoacacia and T. officinale these plants were selected for the present work. Therefore we continue our laboratory work33,34,35 on seed extracts of these plants. Moreover the defatted extracts of worked seeds were screened for antimicrobial activity and functional group analysis.
Table 1: Characteristics of the worked plants
|
S. No. |
Common name |
Botanical name |
Family |
Uses |
|
1 |
Wanwagun |
P. hexandrum Royle |
Berberidaceae |
It possesses anti-tumor and anti-oxidant activities11,12,13 . It is Used in Ayurvedic system in the treatment of diseases like Condylona acuminate, Taenia capitis, monocytoid leukemia, Hodgkin’s diseases, cancer14,15,16. |
|
2 |
Jangli Zeer |
B. persicum (Bioss.) B. |
Apiaceae |
This plant is used as spice and flavouring agent in foods due to its earthy aroma17,18. Seeds are used for treatment of toothache, dyspepsia, diarrhea and jaundice19. |
|
3 |
Shangir |
A. precatorius L. |
Fabaceae |
Its seeds are used for tuberculosis and painful swellings20. It is an excellent source of vitamins, minerals, amino acids and carbohydrates21. It is used as aphrodisiac, laxative, bronchodilator, anti-malarial, anti-convulsant, antioxidant, anti-inflammatory, analgesic22,23,24,25. |
|
4 |
Hand |
T. officinale Weber. |
Asteraceae |
This plant is used as a food26. Its leaf extract is used against obesity and cardiovascular diseases27. It is used as an anti-inflammatory medicine28 and to treat infections, bile, liver problems and diuretic29. |
|
5 |
Charleri |
A. virdis L. |
Amaranthaceae |
Entire plant is used to treat dysentery and inflammation 30. It possesses anti-oxidant activities31. |
|
6 |
Singli Mingli |
D. deltoidea Wall. |
Dioscoreaceae |
This plant is used to treat various digestive disorders such as diarrhea, irritability, abdominal pain32. It is used as traditional medicine and steroidal drugs in western india33. |
|
7 |
Suchal |
M. neglecta Wallr. |
Malvaceae |
Aerial parts of this are used to treat skin afflications and gastrointestinal disorders34. It is used to treat asthma, colds, digestive, urinary and abdominal problems35. |
|
8 |
Kikur |
R. pseudoacacia L. |
Fabaceae |
The plant is used as an antioxidant, anti-spasmodic, febrifuge, diuretic, antitumor, antimicrobial and laxative36. Its leaves are used for treatment of wounds. |
Materials and Methods
Materials and Chemicals
The seeds of all worked plants were collected from northern parts of Kashmir (India) in September-October 2018. The plants were identified by Dr. Sajad Gangoo and Dr. Tahir Mushtaq, faculty at Forest department, SKAUST-K. Seeds were allowed to dry out at room temperature until they get fully dried. All the chemicals and reagents were purchased from Sigma-Aldrich, USA.
The fungal strains used in this study viz., Aspergillus niger (MTCC-281), Aspergillus fumigates (MTCC-343) and Penicellium marneffei (recultured) were obtained from the section of plant pathology, Botany department, Aligarh Muslim University and different strains of bacteria, viz. Esxherichia coli (ATCC-25922) Pseudomonas aeruginosa (PA01) and Staphylococcus aureus (ATCC-29213) were collected from Agricultural microbiology department, Aligarh Muslim University, Aligarh (India).
Soxhlet Extraction of Seeds
The seeds of A. precatorius, A. virdis, B. persicum, D. deltoidea, M. neglecta, P. hexandrum, and R. pseudoacacia and T. officinale were dried, grinded into powder in mixer grinder. The oil was extracted by using 180 ml of petroleum ether (40-60°C) in Soxhlet apparatus (J-SIL Scientific Industries, Agra India). The solvent was evaporated by Rota vapor (Butchi R-300, Mumbai India). The samples were stored at 4oC until further use. Defatted seed cakes of all plants were extracted separately with chloroform and methanol (1/1, v/v). The extracts were then dried over anhydrous Na2SO4, filter and solvent evaporated. These extracts were used for antifungal and antibacterial activities. The extraction time was about 6-8 hours for each extraction. The percentage oil yield (%) of seed oil was calculated. The analytical values of the seed extracts were determined in accordance with AOCS36.
Synthesis of Esters of Fatty Acid (FAMEs)
1g of oil was saponified separately by refluxing with 0.5N potassium hydroxide (KOH) solution prepared in ethanol and the reaction was observed in thin layer chromatography (TLC). After completation of reaction, unsaponifiable matter was separated out with diethyl ether and remained part was acidified with 6N hydrochloric acid (HCl). Finally diethyl ether was used to separate out the mixed fatty acids (MFAs) in separating funnel.
In second step, of the reaction, MFAs are added with an excess amount of absolute methanol with sulphuric acid as a catalyst. The subsequent mixture was diluted to cloud point by ice chilled water in ice bath. The overall mixture was extracted repeatedly with ether. All the ethereal extracts were washed with 5% aqueous sodium bicarbonate and they are passed through anhydrous sodium sulphate to yield (FAMEs). Normal column chromatography was used to purify FAMEs and the eluent used was n-hexane and diethyl ether (98/2, v/v). The FAMEs were identified by Fourier transform infrared spectroscopy (FTIR) as given in (Figure S1 Supplementary material).
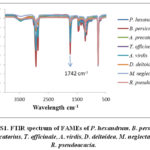 |
Figure S1: FTIR spectrum of FAMEs of P. hexandrum |
Chromatography
TLC was carried on thin (0.5mm) plates of glass (10 cm × 5 cm) dimensions, which are polished with uniform, layer of silica gel (60-120, Merck, Mumbai, India) formed in ethanol. Petroleum ether/diethyl ether/acetic acid (80/20/1, v/v) were used as developer in TLC analysis in iodine vapor chamber. The separation of FAMEs was done by column chromatography with silica gel (Merck, Mumbai, India, 60-120 mesh).
Instrumentation
Fourier Transform Infrared Spectrometer (Perkin–Elmer,UK) fitted with zinc selenide crystal was used for functional group analysis. FAMEs were analyzed by using Gas chromatography (GC, Clarius-680, Perkin Elmer) with flame ionization (FID) and mass spectrometer (MS) as its detectors. Helium was used as a carrier gas, at a flow rate of 0.5 mL/min through an Elite-5MS (0.25mm × 30mm) capillary column. Oven temperature was initially 180°C for 2min, raised to 200°C for 2°C/min, to a final temperature of 215°C at 2°C/min. injector and detector temperatures are set at 180oC and 280oC respectively.
Determination of Antimicrobial Activity
Antifungal Assay by Disc Diffusion Technique
The defatted seed extracts were studied for antifungal activity against A. fumigatus, A. niger and P. Marneffi by disc method as per clinical laboratory standards (NCCLS) on filamentous fungi diffusion37. The fungal cultures were developed on czapexdox broth (diffco). Twenty ml of agar media was taken into petri dishes and stand to solidify. The lawning of individual fungal strain was prepared on the surface of agar media around the disc. The sterilized discs (6mm in Whatman paper, 42) were transferred in added standard (S, S/2 and S/4) concentrations. A disc without seed extract was used as negative control while as standard nystatin drug was used as positive control. The mycelium mats of A. niger, A. fumigatus and P. marneffi of old culture (7- day) were washed carefully and kept in normal saline solution. Finally they are filtered aseptically through wool glass. The inoculum was adjusted to 1-5 × 100 ml, and colony forming units/mL of suspension of test fungi were determined out. The conidial appearance was used for in-vitro antifungal assay tests. All the tests were repeated (×3). The dishes were incubated at for 48 h at a temperature of 28ºC and inhibition zones of fungus (mm) were determined.
Antibacterial Activity by Broth Dilution Method
The antibacterial activity of defatted seed extracts against two gram positive E. coli, S. aureus and gram negative P.aeruginosa bacteria was done to determine the lowest concentration which inhibit growth entirely also called Minimal Inhibitory Concentration (MIC) by broth dilution method38 with a concentration of 104 CFU. Initially the samples were diluted in Muller Hinton medium with a concentration 500 µg/ml, which were serially and subsequently diluted to attain 250, 125, 62.5 and 31.25 µg/ml concentrations. Ten microliters of bacterial cultures were added to each. After 20 hours of incubation at 370C.
Statistical Analysis
Antimicrobial observations found were statistically evaluated by using analysis of variance (ANOVA) by using the IBM SPSS Statistics 20 software. Duncan’s post hoc tests were performed to observe the differences between groups at 5% significance level.
Results and Discussion
Physicochemical Properties
Saponification value (SV) is a good tool for the determination of average molecular weight of fatty acid in a triglyceride39. Lower the SV of the oil the large is the chain length of triglyceride and hence molecular weight. From Table 2 the highest saponification values was shown by B. persicum and R. pseudoacacia followed by T. officinale, A. precatorius, D. deltoidea, M. neglecta, P. hexandrum and A. virdis. Iodine value gives the idea of degree of unsaturation. As listed in Table 2 R. pseudoacacia, T. officinale and M. neglecta shows maximum iodine values followed by B. persicum, D. deltoidea, P. hexandrum and A. virdis respectively.
Table 2: Oil % (w/w), S.V and I.V of P. hexandrum, B. persicum, A. precatorius, T. officinale, A. virdis, D. deltoidea, M. neglecta and R. pseudoacacia seed oils.
| Plants | Oil percentage (w/w) | Saponification value | Iodine value |
| P. hexandrum | 6.9 | 120.60 | 59.88 |
| B. persicum | 6.83 | 177.70 | 93.90 |
| A. precatorius | 16.78 | 122.19 | 56.93 |
| T. officinale | 3.8 | 127.49 | 109.83 |
| A. virdis | 2.38 | 117.52 | 53.98 |
| D. deltoida | 1.6 | 121.13 | 89.20 |
| M. neglecta | 17.19 | 121.13 | 109.47 |
| R. pseudoacacia | 8.44 | 151.70 | 161.90 |
Fatty Acid Composition
The composition of fatty acid was determined by gas chromatography (GC) and individual fatty acids were identified by mass spectroscopy (MS) as given in (Figure S2 to Figure S16 Supplementary material). Fatty acid composition of all seed oils of plants are shown in Table 3. The seed oils were mostly dominant in unsaturated acids with (62.7%, 84.6%, 64.0%, 65.8%, 60.9%, 62.2%, 62.6% and 77.5%) composition of total unsaturated fatty acids (TUFA) for P. hexandrum, B. persicum, A. precatorius, T. officinale, A. virdis, D. deltoidea, M. neglecta and R. pseudoacacia respectively. Oleic acid was the most dominant monounsaturated fatty acid in A. precatorius, A. virdis, P. hexandrum and D. deltoidea with (60.2%, 58.9%, 57.5% and 21.7%) respectively, while as it is found in smaller amounts in T. officinale 6.0%, M. neglecta 3.7% and R. pseudoacacia 1.3%. The dominant amount 64% petroselinic acid was found in seed oil of B. persicum. Among polyunsaturated fatty acids, linoleic acid was most dominant in T. officinale, M. neglecta, R. pseudoacacia, D. deltoidea and B. persicum with (59%, 57.4%, 45.6%, 39.3% and 15.9%) respectively, while it is found in minor amount in P. hexandrum 3.7% and A. virdis 0.9%. The linolenic acid was dominant in R. pseudoacacia 30.6% while low percentage in P. hexandrum 1.1%, A. precatorius 0.6% and M. neglecta 0.7%. Among SFAs palmitic and stearic acid were the main fatty acids found. Palmitic acid includes (25.6%, 22.7%, 20.2%, 14.9%, 11.1%, 11.0%, 8.1% and 3.9%) for M. neglecta, P. hexandrum, D. deltoidea, A. virdis, A. precatorius, T. officinale, B. persicum and R. pseudoacacia respectively and stearic acid includes (10.7%, 8.1%, 4.8%, 4.5%, 3.5%, 3.5% and 1.2%) for P. hexandrum, M. neglecta, T. officinale, A. precatorius, A. virdis, D. deltoidea and R. pseudoacacia respectively. The fatty acid composition of P. hexandrum is quiet similar to that of Sterculia tomentosa from Sudan40. In B. persicum and R. pseudoacacia the major fatty acids found are comparatively similar in composition as reported in Olea europea from Palestine and Temperate growing Cannabis sativa respectively41,42. The fatty acid composition of D. deltoidea is similar to cotton seed which is used in pharmaceutical and cosmetic preparation43.
 |
Figure S2: Mass spectrum of Methyl tetradecanoate. |
 |
Figure S3: Mass spectrum of Pentadecanoic acid, methyl ester. |
 |
Figure S4: Mass spectrum of Hexadecanoic acid, methyl ester. |
 |
Figure S5: Mass spectrum of 9-Hexadecenoic acid, methyl ester. |
 |
Figure S6: Mass spectrum of 10-Heptadecenoic acid, methyl ester. |
 |
Figure S7: Mass spectrum of Methyl stearate. |
 |
Figure S8: Mass spectrum of Petroselinic acid, methyl ester. |
 |
Figure S9: Mass spectrum of 9-octadecenoic acid, methyl ester. |
 |
Figure S10: Mass spectrum of 9,12-octadecadienoic acid, methyl ester. |
 |
Figure S11: Mass spectrum of 9,12,15-octadecatrienoic acid, methyl ester. |
 |
Figure S12: Mass spectrum of Eicosanoic acid, methyl ester. |
 |
Figure S13: Mass spectrum of 11-Eicosenoic acid, methyl ester. |
 |
Figure S14: Mass spectrum of Docosanoic acid, methyl ester. |
 |
Figure S15: Mass spectrum of 13-Docosenoic acid, methyl ester. |
 |
Figure S16: Mass spectrum of Tetracosanoic acid, methyl ester. |
Table 3: Fatty acid composition of seed oils of P. hexandrum, B. persicum, A. precatorius, T. officinale, A. virdis, D. deltoidea, M. neglecta and R. pseudoacacia.
|
Common and systematic names |
Area (%) |
|||||||
|
P. hexandrum |
B. persicum |
A. precatorius |
T. officinale |
A. virdis |
D. deltoida |
M. neglecta |
R. pseudoacacia |
|
|
Myristic acid14:0 |
0.4 |
– |
– |
– |
0.6 |
1.2 |
0.5 |
– |
|
Pentadecanoic acid 15:0 |
– |
– |
– |
– |
1.1 |
– |
– |
– |
|
Palmitic acid 16:0 |
22.7 |
8.1 |
11.1 |
11.0 |
14.9 |
20.2 |
25.6 |
3.9 |
|
Palmitoleic acid16:1 |
– |
– |
0.8 |
0.5 |
0.7 |
0.3 |
– |
|
|
Heptadecenoic acid 17:1 |
– |
– |
– |
– |
– |
– |
0.5 |
– |
|
Stearic acid 18:0 |
10.7 |
– |
4.5 |
4.8 |
3.5 |
3.5 |
8.1 |
1.2 |
|
Petroselinic acid 18:1 ∆6 |
– |
64.0 |
– |
– |
– |
– |
– |
– |
|
Oleic acid 18:1 ∆9 |
57.5 |
– |
60.2 |
6.0 |
58.9 |
21.7 |
3.7 |
1.3 |
|
Linoleic acid 18:2 |
3.7 |
15.9 |
– |
59.0 |
0.9 |
39.3 |
57.4 |
45.6 |
|
Linolenic acid 18:3 |
1.1 |
– |
0.6 |
– |
– |
– |
0.7 |
30.6 |
|
Arachidic acid 20:0 |
0.4 |
0.7 |
1.4 |
3.1 |
1.4 |
0.9 |
– |
– |
|
Gondoic acid 20:1 |
– |
1.5 |
2.6 |
– |
0.6 |
0.5 |
– |
– |
|
Behenic acid 22:0 |
– |
0.6 |
7.1 |
2.3 |
– |
2.3 |
– |
– |
|
Erucic acid22:1 |
– |
3.2 |
0.6 |
– |
– |
– |
– |
– |
|
Lignoceric acid24:0 |
– |
0.3 |
3.5 |
– |
– |
1.2 |
– |
– |
|
Total unsaturated acids |
62.7 |
84.6 |
64.0 |
65.8 |
60.9 |
62.2 |
62.6 |
77.5 |
|
Total saturated acids |
33.8 |
9.7 |
27.6 |
21.2 |
21.5 |
29.3 |
34.2 |
5.1 |
Functional Group Analysis by FTIR
Transmission spectra of defatted seed extracts of P. hexandrum, B. persicum, A. precatorius, T. officinale, A. virdis, D. deltoidea, M. neglecta and R. pseudoacacia are shown in (Figure S17 Supplementary material) and peak values are given in (Table 4). The IR peaks for B. persicum seed extracts are pointed out while as IR spectrum of rest of seed extracts are given in (Figure S17 Supplementary material). The presence of various functional groups as depicted by IR confirm various active substances present defatted seed extracts of these plants which could be responsible for their biological activities.
 |
Figure S17: FTIR analysis of the defatted seed extracts of P. hexandrum |
 |
Table 4: Regions of FTIR spectrum of defatted seed extracts of P. |
Table was constituted according to References45,46,47.
Antifungal Activity of Defatted Seed Extracts
All the defatted seed extracts showed good antifungal activity at all concentrations, but most dominant activity was shown at Standard solution S (Table 5). The defatted seed extracts of T. officinale, B. persicum and M. neglecta displayed most dominant effect with zone of inhibition 19.53 mm, 18.82 mm and 18.61 mm respectively against A. niger with respect to nystatin 20.36 mm. Against A. fumigates the inhibition zone was found to be 21.42 mm, 21.06 mm and 21.05 mm for T. officinale, B. persicum and M. neglecta respectively with nystatin displayed 25.56 mm inhibition zone. Against P. morneffei the most dominant effect was shown by T. officinale 24.96 mm, M. neglecta 24.22 mm followed by R. pseudoacacia 23.93 mm, B. persicum 23.81 mm, A. precatorius 22.46 mm with respect to nystatin 28.35 mm (Table 5).
 |
Table 5: Antifungal activity of extracts of defatted seeds of P. |
Antibacterial Activity of Defatted Seed Extracts
The results of this study are shown in (Table 6). The defatted seed extracts of B. persicum, A. precatorius and M. neglecta displayed lowest MIC 125 µg/ml, while other defatted extracts showed MIC 250 µg/ml except R. pseudoacacia which showed 500 µg/ml. Inhibition against S. aureus defatted seed extracts of A. precatorius, A. virdis and D. deltoidea showed lowest MIC 125 µg/ml, while defatted extracts of other plants (Table 6) showed MIC 250 µg/ml except R. pseudoacacia showed 500 µg/ml. Against P. aeruginosa (gram negative bacteria) defatted seed extracts of B. persicum, T. officinale, D. deltoidea and M. neglecta showed lowest MIC 125 µg/ml while other seed extracts of plants showed MIC 250 µg/ml.
Table 6: Antibacterial activity of extracts of defatted seeds of P. hexandrum, B. persicum, A. precatorius, T. officinale, A. virdis, D. deltoidea, M. neglecta and R. pseudoacacia against E. coli, S. aureus and P. aeruginosa.
|
S. No. |
Seed extracts |
Minimal Inhibitory Concentration (µg/ml) |
||
|
E. coli |
S. aureus |
P. aeruginosa |
||
|
1 |
P. hexandrum |
250 |
250 |
250 |
|
2 |
B. persicum |
125 |
250 |
125 |
|
3 |
A. precatorius |
125 |
125 |
250 |
|
4 |
T. officinale |
250 |
250 |
125 |
|
5 |
A. viridis |
250 |
125 |
250 |
|
6 |
D. deltoidea |
250 |
125 |
125 |
|
7 |
M. neglecta |
125 |
250 |
125 |
|
8 |
R. pseudoacacia |
500 |
500 |
250 |
|
9 |
Streptomycin |
5 |
5 |
10 |
Conclusion
From the above results, this research concluded that these plants contains high percentage of oleic, linoleic and significant amount of palmitic acids. Moreover the defatted seed extracts showed powerful antifungal and antibacterial activities. Based on above results we conclude that seed extracts of these plants could be used in food industry, cosmetic industry and source of new natural fungicide and bactericide in pharmaceutical industry. However further work need to be carried on isolation, purification and characterization of active compounds in defatted seed extracts.
Conflict of Interest
The authors proclaim no conflict interest.
Acknowledgement
We are grateful to University Sophisticated Instrumentation Facility (USIF), and instrumentation faculty in Chemistry department, A.M.U, Aligarh for providing the required facilities to complete out this work. Prof. Sajad and Dr. Tahir are duly acknowledged for helping and guiding in collection and identification of seeds.
References
- Sharififar, F.; Yassa, N.; Mozaffarian, V. Pak. J. Pharma. Sci. 2010, 23, 300-304.
- Arora, R.; Gill, N. S.; Kaur, S.; Jain, A. D. J. Pharmacol. Toxicol. 2011, 6, 580-588.
CrossRef - Escudero, N. L.; De Arellano, M. L.; Fernández, S.; Albarracín, G.; Mucciarelli, S. Plant Foods Human Nut. 2003, 58, 1-10.
CrossRef - Gowdey, G.; Lee, R. K.; Carpenter, W. M. Oral Pathol.Oral Radio. Endodon. 1995, 79, 64-67.
CrossRef - Lewis, W. H. Plants used medically by indigenous peoples. In Phytochem. Res. Med. Agri. 1992, (pp. 33-74). Springer, Boston, MA.
CrossRef - Rajaram, N.; Janardhanan, K. Plant Foods Human Nut. 1992, 42, 285-290.
CrossRef - Choi, U. K.; Lee, O. H.; Yim, J. H.; Cho, C. W.; Rhee, Y. K.; Lim, S. I.; Kim, Y. C. Int. J. Mol. Sci. 2010, 11, 67-78.
CrossRef - Ahmed, S. A.; Hanif, S.; Iftkhar, T. Open J. Med. Microbio. 2013, 3, 164-171.
CrossRef - Jain, S. K. 2nd Revised Edition, National Book Trust, New Delhi, India. 1975, 1-180.
- Mayer, M.; Zbinden, M.; Vogl, C. R.; Ivemeyer, S.; Meier, B.; Amorena, M.; Maeschli, A.; Hamburger, M.; Walkenhorst, M. J. Ethnobio. Ethnomed. 2017, 13, 1.
CrossRef - Okoko, I. I.; Osinubi, A.; Olabiyi, O.; Kusemiju, T.; Noronha, C.; Okanlawon, A. Endo. Pract. 2010, 16, 554-560.
CrossRef - Mensah, A. Y.; Bonsu, A. S.; Fleischer, T. C. Int. J. Pharm. Sci . Rev. Res. 2011, 6, 9-13.
- Kumar, A.; Lakshman, K.; Jayaveera, K. N.; Nandeesh, R.; Manoj, B.; Ranganayakulu, D. Arch. Bio. Sci. 2010, 62, 185-189.
CrossRef - Sofi, P. A.; Zeerak, N. A.; Singh, P. Turk. J. Bio. 2009, 33, 249-258.
CrossRef - Sultana, S.; Ripa, F. A.; Hamid, K. Pak. J. Bio. Sci. 2010, 13, 340-343.
CrossRef - Subhash, C.; Sarla, S.; Mridul, D. Int. J. Res. Pharm. 2012, 3, 152-156
- Mayer, M.; Zbinden, M.; Vogl, C. R.; Ivemeyer, S.; Meier, B.; Amorena, M.; Maeschli, A.; Hamburger, M.; Walkenhorst, M. J. Ethnobio. Ethnomed. 2017, 13, 1.
CrossRef - Jackson, D. E.; Dewick, P. M. Phytochem. 1984, 23, 1147-1152.
CrossRef - Canel, C.; Dayan, F. E.; Ganzera, M.; Khan, I. A.; Rimando, A.; Burandt Jr, C. L.; Moraes, R. M. Planta Med. 2001, 67, 97-99.
CrossRef - Moraes, R. M.; Bedir, E.; Barrett, H.; Burandt Jr, C.; Canel, C.; Khan, I. A. Planta Medica. 2002, 68, 341- 344.
CrossRef - Cobb, M. W. J. Amer. Acad. Dermatol. 1990, 22, 547-566.
CrossRef - Beutner, K. R. In Sem. Dermatol. 1990, 9,148-151.
- Rosu, A. F.; Bita, A.; Calina, D.; Rosu, L.; Zlatian, O.; Calina, V. Eur. J. Hospit. Pharm. Sci. Pract. 2012, 19, 216-216.
CrossRef - Mir, M. A.; Sawhney, S. S.; Jassal, M. S. Amer. J. Adv. Drug Deliv. 2015, 3, 160-180.
- Schutz, K.; Carle, R.; Schieber, A. J. Ethnopharmacol. 2006, 107, 313-323.
CrossRef - Lopez‐Miranda, J.; Badimon, L.; Bonanome, A.; Lairon, D.; Kris‐Etherton, P. M.; Mata, P.; Perez‐Jimenez, F. Nut. Rev. 2006, 64, S2-S12.
CrossRef - Chen, S. H.; Chuang, Y. J. Analytica Chimica Act. 2002, 465, 145-155.
CrossRef - Soybean, I. Advan. Agronom. 2004, 84, 273-306.
CrossRef - Li, X. M.; Luo, X. G.; Si, C. L.; Wang, N.; Zhou, H.; He, J. F.; Zhang, T. C. Eur. J. Med. Chem. 2015, 96, 436-444.
CrossRef - Valle Jr, D. L.; Andrade, J. I.; Puzon, J. J. M.; Cabrera, E. C.; Rivera, W. L. Asian Pacific J. Trop. Biomed. 2015, 5, 532-540.
CrossRef - Zampini, I. C.; Vattuone, M.A.; Isla, M. I. J. Ethnopharmacol. 2005, 102, 450-456.
CrossRef - Chitwood, D. J. Annu. Rev. Phytopathol. 2002, 40, 221-249.
CrossRef - Nengroo, Z. R.; Rauf, A. Ind. Crops Prod. 2019,140, 111596.
CrossRef - Parveen, H.; Rauf, A. Ind. Crops Prod. 2008, 27, 118-122.
CrossRef - Sharma, S.; Gangal, S.; Rauf, A. Ind. Crops Prod. 2009, 30, 325-328.
CrossRef - Link, W. E. Off. Amer. Oil Chem. Soc. 1973, Methods Da 15-48 and Da 16-48.
- Bauer, A. W.; Kirby, W. M. M.; Sherris, J. C.; Turck, M. Amer. J. Clin. Pathol. 1966, 45, 493-496.
CrossRef - Wiegand, I.; Hilpert, K.; Hancock, R. E. Nat. Protocol. 2008, 3(2), 163.
CrossRef - Nehdi, I. A.; Sbihi, H.; Tan, C. P.; Zarrouk, H.; Khalil, M. I.; Al-Resayes, S. I. Ind. Crops Prod. 2012, 36, 54-58.
CrossRef - Henry, A. J.; Grindley, D. N. J. Soc. Chem. Ind. 1944, 63, 188-190.
- Da Porto, C.; Decorti, D.; Tubaro, F. Ind. Crops Prod. 2012, 36(1), 401-404.
CrossRef - Lodolini, E. M.; Polverigiani, S.; Ali, S.; Mutawea, M.; Qutub, M.; Arabasi, T.; Pierini, F.; Abed, M.; Neri, D. J. Oleo Sci. 2017, 16184.
- Alvarez, A. M. R.; Rodríguez, M. L. G. Grasas Aceite. 2000, 51, 74-96.
- Guillen, M. D.; Cabo, N. J. Am. Oil Chem. Soc. 1997, 74, 1281-1286.
CrossRef - Silverstein, R. M.; Bassler, G. C.; Kiemle, D. J. Hoboken, USA: John Wiley & Sons. 2005, (Chapter 2).
- Vlachos, N.; Skopelitis, Y.; Psaroudaki, M.; Konstantinidou, V.; Chatzilazarou, A.; Tegou, E. Anal. Chim. Act. 2006, 573, 459-465.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









