Efficient Electrochemical Removal of Ammonium in Wastewater Using Various Cathodes and A Ti/IrO2 Anode
Alfa-Sika Mande Seyf-Laye1,2,3* , Akpataku Kossitse Venyo2,3, Tchakala Ibrahim2, Djaneye-Boundjou Gbandi3, Bawa Leman Moctar2 and Chen Honghan1
, Akpataku Kossitse Venyo2,3, Tchakala Ibrahim2, Djaneye-Boundjou Gbandi3, Bawa Leman Moctar2 and Chen Honghan1
1Beijing Key Laboratory of Water Resources & Environmental Engineering, China University of Geosciences (Beijing), Beijing 100083, P.R. China
2Laboratory of Applied Hydrology and Environment, University of Lome, BP. 1515, Togo
3Faculty of Science and Technology, University of Kara, BP. 404, Togo
Corresponding Author E-mail: seyf009@yahoo.ca
DOI : http://dx.doi.org/10.13005/ojc/360122
Article Received on : 04 Aug 2018
Article Accepted on : 13 Feb 2020
Article Published : 21 Feb 2020
The removal of ammonium in wastewater by an electro-oxidation method using Cu, Fe and alloy Cu/Zn electrodes as cathodes and Ti/IrO2 as anode were investigated. The effects of the various operating parameters, including, electro-oxidation duration, temperature, salt (NaCl) and electrode types on the ammonium removal efficiency from synthetic wastewater and real wastewater in batch EC process, were also investigated. The results showed that the maximum removal efficiency was achieved at a current density of 16mA/cm2, at a pH of 5.1, temperature of 30°C and 0.5 g/L NaCl. Among tested materials, Cu/Zn cathode showed the highest ammonium removal rate. The results revealed that the ammonium was removed and 0.2 mg/L of nitrate and 0.023 mg N/L of nitrite were found. Finally, the really wastewater was tested at 40°C in presence of Cu/Zn cathode/ Ti/IrO2 anode with a current density of 18.32 mA/cm2 and the result indicates that electro-oxidation is very efficient and was able to achieve ammonium removal (99.99 %), and COD removal (38 %) in 30 min. During the treatment concentrations of nitrate, nitrite and phosphorous were not detected in the effluent after 30 min. The ammonia removal followed pseudo-first-order kinetics. The electrochemical process can be applied successfully as a final polishing step, or as an alternative method to biological nitrification.
KEYWORDS:Electrochemical; Electrodes; Nutrient Removal; Ti/IrO2; COD
Download this article as:| Copy the following to cite this article: Seyf-Laye Alfa-Sika M , Venyo A. K, Ibrahim T, Gbandi Djaneye-Boundjou, Moctar B. L, Honghan C. Efficient Electrochemical Removal of Ammonium in Wastewater Using Various Cathodes and A Ti/IrO2 Anode. Orient J Chem 2020;36(1). |
| Copy the following to cite this URL: Seyf-Laye Alfa-Sika M , Venyo A. K, Ibrahim T, Gbandi Djaneye-Boundjou, Moctar B. L, Honghan C. Efficient Electrochemical Removal of Ammonium in Wastewater Using Various Cathodes and A Ti/IrO2 Anode. Orient J Chem 2020;36(1). Available from: https://bit.ly/37I4HWd |
Introduction
Water pollution by nutrients is a historical and ever-growing concern in developed and developing countries alike. The ammonium in the water was usually generated from a process of incomplete degradation of organic matter. Ammonium was generated from the reaction of iron-containing minerals with nitrates. This was an excellent indicator of water pollution polluted by organic waste that came from agricultural, domestic or industrial. High concentration ammonium could promote eutrophication, which was fatal to aquatic life and an obstacle to the disinfection of water sources (Kim et al., 2005; Pérez et al., 2012a). Ammonium is not toxic, but could cause several problems such a decrease in the efficiency of the chlorine treatment and the development of microorganisms responsible for flavors and odors. The oxidation of ammonium would produce nitrite, which could be oxidized to nitrate. Meanwhile, nitrate could cause serious health problems in humans such as the “blue baby syndrome” in infants, liver damage and cancer (Gupta et al., 2000, Shrimali et al., 2001). The main reason for the increase in nitrate in groundwater was mainly attributed to the abusive use of nitrogen fertilizer (Martin, 1979). Nitrate in the solution had some properties such as high solubility, less capacity for precipitation and adsorption, so it was difficult to remove nitrate from solution. Thus, electrochemical methods have been evaluated for this purpose (Arroyo,et al., 2009, Nouri et al., 2010) and several electrodes such as Cu, Fe, Zn, Pt/Ir and Au. However, some electrodes such as Cu and Fe were recognized as promoters for the nitrate reduction. The aim of this work was to find the best conditions to reduce the concentration of ammonium.
Experimental Part
Material
Only analytically graded chemicals were used without further treatment. Stock ammonium solutions were prepared with distilled water from ammonium chloride.
Analytical Method
At given time intervals, liquid samples were withdrawn from the feed tank. Ammonium concentrations were determined by a flow injection analyzer (Lachat-QC8000, Hach, Loveland, CO, USA) using a salicylate spectrophotometric method. Nitrate ( NO3– – N ), nitrite ( NO2– – N ) and phosphorous concentrations were determined by ion chromatography (DX-600, DIONEX Co., Sunnyvale, USA). The detection limit for each analyte was 0.1 mg/L; the standard deviation for replicate samples was ±10%. The portable multiparameter meters (HQ40d, Hach, Loveland, CO, USA) was used to measure pH while COD was analyzed according to the APHA methods (APHA, 1998).
Experimental Setup
A 613 mL dacryl plastic rectangular tank, with dimensions of 6.5 × 6.5 cm square base and a height of 14.5 Cm with two plate electrodes (1 anode and 1 cathode) with an area of 6 × 14 cm2 was used as shown in Fig(1). The plates were placed vertically in the tank and separated by a distance of 2 Cm. Cu, Fe and Cu/Zn plates were used as a cathode while Ti/IrO2 plates were used as anode. Alternating electrodes were connected to a digital DC power supply (containing amperemeter and voltmeter was used to measure the current intensity passing through the circuit and the applied potential difference respectively), characterized by 10 A for current intensity and a range of 0–40 V for voltage in monopolar mode. Synthetic ammonium solutions (with concentration of 50 mg N/L) were used. At the beginning of each run the ammonium solution of 50 mg N /L concentration was fed into the cell and solution of 0.5g/L NaCl (as electrolyte) was added to it to increase the solution conductivity. Each run was timed starting with the DC power supply switching on. Thermostatic bath shaker was used to set the temperature. Finally, the better material electrode was used to treat the real wastewater.
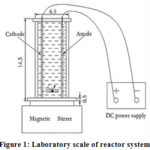 |
Figure 1: Laboratory scale of reactor system |
Electrocoagulation Procedure
A volume of 300 mL of synthetic waste solution was used for each run and mixed with an appropriate concentration of NaCl which was used to increase the conductivity. The prepared solutions were put into an electrolytic cell. NaOH or HCl solutions were used to adjust the pH of the synthetic waste solution. Direct current was used for an electrolysis run during 60 minutes via two electrodes. At every 10 minutes interval, a volume of 10 mL of the solution was sampled and filtered for the determination of residual concentrations of NH4+, NO3– and NO2–. The location of the drawn samples was kept constant for each run. Before each run, the electrodes as cathode were cleaned with sandpaper to remove impurities and toxic layer, and then were washed with 15% w/v of HCl solution in order to remove any adhering scales or oxides. After this process, all the electrodes were cleaned with distilled water and dried until the electrodes are used again.
Results and Discussion
Effect of Different Electrodes on Ammonium Removal Without NaCl
As shown in Fig. 2, the electrolysis was carried out when used different electrodes such as Cu; Fe and alloy Cu/Zn as cathode without adding NaCl, the ammonium removal efficiency was 94.4%, concentrations of NO3– – N was 0.53 mg N/L and NO2– – N. It was probably because Cu could inhibited the hydrogen evolution and its seemed that Cu had more negative potential that was approximately 1.2 V which could inhibit the amount of hydrogen production, and then affected the ammonium removal and nitrate formation (Reyter et al., 2008, Szpyrkowicz et al., 2006), the phenomenon was because less hydrogen would lead to the low selectivity of ammonium, and then Cu electrode was difficult to subside in the solution.
The ammonium removal efficiency was 98.7% with the Fe as electrode. The higher removal with iron electrode could be due to the greater donating electron. Nitrite-N was an intermediate product and could be oxidized to nitrate. NO2– – N concentration with Fe and Cu as electrodes was lower and the reduction of ammonium to nitrate was relatively higher. Because the reaction was slow during the early 30 minutes due to that the presence of ferrous ions would affect the reaction when the concentration of dissolved oxygen was low in the solution. When the reaction was carried out in 40 min, ferrous compounds were oxidized at a rate that was enough to precipitate hydrated ferric hydroxide at a higher oxygen concentration. Meanwhile, the nitrate concentration sharply increased to 1.43 mg N/L and decreased finally (Fig 2). The utilization of Ti/IrO2-Pt as anode favored considerably elimination of ammonium due to the high oxidation ability. In the present experiment, Fe cathode was more useful for ammonium removal and nitrate reduction than Cu electrode without NaCl.
Ammonium removal efficiency was 99.28% using alloy Cu/Zn as cathode (Fig2). The removal efficiency of ammonium and the formation of nitrate increased significantly at the first time and finally decreased. The mechanism of electrolytic ammonia reduction was complex. Without NaCl addition, the oxidation of ammonium and accumulation of nitrate during electrolytic process could be attributed to the alloy Cu/Zn cathode prossessed good corrosion resistance, and Zinc had good electro-activity and Cu exhibited good activity of electro-reduction. As a result that high ammonium removal efficiency was expected when using the alloy Cu/Zn as electrode. According to the the research of (Yaning et al.2012), the ammonium removal efficiency was better than using Cu/Zn as electrode. The nitrate concentration obtained from ammonium oxidation was 1.1 mg N/L. According to the results, it could be concluded that Cu/Zn as cathode was more suitable than Fe and Cu in the absence of NaCl.
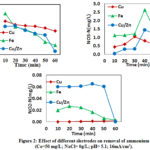 |
Figure 2: Effect of different electrodes on removal of ammonium |
Effect of Different Electrodes on Ammonium Removal with NaCl
The elimination of ammonium and formation of nitrite and nitrate was shown in Fig. 3 during electrolysis using Cu, Fe and alloy Cu/Zn as cathode in the presence of 0.5 g/L NaCl. The ammonium removal efficiency was 97.44%, then 0.053 mg N/L and 2.5 mg N/L nitrite and nitrate were formed in 60 min when using Cu electrode respectively. While ammonium was completely remove in 40 min and 50 min using respectively Cu/Zn and Fe electrodes. Comparing to the result obtained by Fe and Cu/Zn cathodes, the ammonium removal efficiency was lower and the nitrate concentration in treated solution was higher with Cu electrode than those with Fe and Cu/Zn cathode. Nitrite concentration was lower with Fe electrode and higher with Cu and Cu/Zn electrodes. This could be explained by the fact that the Cu/Zn electrode had a higher potential which would promote hydrogen evolution reaction in the vicinity of cathode. As shown in Fig. 3, it was clearly that ammonium removal rate greatly increased with the addition of NaCl. It was probably due to the relatively high oxidation ability of the Ti/IrO2–Pt anode used in the present study, with which more hypochlorite ion was formed during electrolysis. In addition, the fact that the iron would donate large number of electrons would promote the electrolysis system for removal of ammonium and nitrite-N and nitrate-N.
In the presence of chloride ion, the hypochlorite ion was formed and then oxide ammonium to nitrite or nitrate. Hence, the indirect oxidation of ammonium played an importance role during the electrochemical method (Deng et al., 2007). The concentration of nitrate sharply increased to 0.28 mg N/L until 40 min and finally decreased, whereas the concentration of nitrite was 0.046 mg N/L using Cu/Zn electrodes. The results were not consistent with the previous study which concluded that the presence of chloride ion in the solution did not change the essential nature of the nitrate reduction.
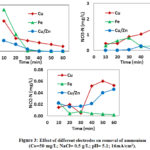 |
Figure 3: Effect of different electrodes on removal of ammonium |
Effect of Temperature on Removal of Ammonium
Cu Electrode
It was confirmed that removal efficiency of ammonium was kept at 98 % whereas the formation of nitrate sharply increased along with the temperature increased from 20 °C to 40 °C (Fig 4). The ammonium removal rate was lowest with Cu, while that was highest with Fe and Cu/Zn cathode. This could be explained at the first by the fact that the copper slowly dissolved in the ammonium solution containing oxygen. Secondly, the previous studies have shown that the voltammetry study of Cu would produce more negative potential and finally would make less evolution of hydrogen (Reyter et al., 2008, Spyrkowicz et al., 2006). That peak would be attributed to that the oxidation/reduction of Cu2O to Cu species. Our result (Li et al., 2009) have also found that copper was less selective for the ammonium removal. However, the products of ammonium like nitrite and nitrate were formed during electrolysis process. The concentration of nitrate produced from ammonium was 0.035, 2.05 and 1.27 mg N/L with 20 °C, 30 °C and 40 °C respectively in 60 minutes (Fig.4). The reason was due to the low pH found at the end of the experiments. Low concentration of nitrite was observed.
 |
Figure 4: Effect of Temperature on removal of ammonium using Cu electrode |
Fe Electrode
The results showed the ammonium removal efficiencies were 98.8%, 99.6% and 98.92% in 20 min when temperature were 20 °C, 30 °C and 40 °C, respectively (Fig. 5). The effect of 30 °C on ammonium removal was found to be better than those of 20 °C and 40 °C because the formation of nitrate was significant when the temperature were 20 °C and 40 °C during the 50 min, and then decreased. This phenomenon could be explained by the fact that iron was corroded due to the increase in pH in the solution. The production of hydrogen gas and ferrous hydroxides raised the pH, which continued until entire solubility of ferrous hydroxide and deposition of iron hydroxide on the surface of the metal as a film form smothering the corrosion reaction. The results explained why (Rajic et al., 2017) found faster nitrate removal rate for oxide coated Fe0. Ammonium was completely removed in 30 min. This could be explained by the fact that the final pH was 6.0, 7.18 and 7.07 at 20 °C, 30 °C and 40 °C, respectively. The oxidation of ammonium to ammonia gas with iron electrode was due to the high pH (Kim et al., 2006). The nitrate concentration was 1.87 mg N/L, 1.69 mg N/L and 1.84 mg N/L at 20 °C, 30 °C and 40 °C, respectively (Fig.5). That demonstrated the nitrate concentration increased with the decrease of temperature. Increasing the temperature resulted in the increase of chloride ion, which indicated the presence of anions could inhibit the reduction of nitrate because that would decrease the active area of the electrode surface (Lee et al., 2002; Pérez et al., 2012b). The concentration of nitrite was detected 0.040 mg N/L, 0.046 mg N/L and 0.039 mg N/L at 20 °C, 30 °C and 40 °C, respectively (Fig. 5). The presence of nitrite increased the catholic current, which indicated that the nitrite reduction rate with iron electrode was faster than nitrate reduction rate.
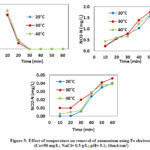 |
Figure 5: Effect of temperature on removal of ammonium using Fe electrode |
Cu/Zn Electrode
According to the results, the ammonium removal efficiencies were 98.08%, 100% and 99.5% in only 20 min at 20 °C, 30 °C and 40 °C, respectively (Fig. 6). The percentage of ammonium decreased when temperature increased from 20 °C to 40 °C. This could be explained by the changes of pH in the different reactions during the oxidation of ammonium. The final pH was 7.13, 7.05 and 7.08 at 20 °C, 30 °C and 40 °C, respectively. These results were in accordance with previous studies that reveal the optimum pH was between 7 and 9 (Lin and Wu, 1996; Chiang et al., 1995, Li and Liu, 2009). It appeared that oxidation of ammonium was strong under alkaline conditions due to that ClO3– agent had a lower oxidability. This explained why the elimination of ammonium was faster at 30 °C and the reaction occurred in low pH value that favored HOCl as agent oxidant. The concentration of nitrite detected throughout the experiment was 0.045 mg N/L, 0.032 mg N/Land 0.023 mg N/L at temperatures of 20 °C, 30 °C and 40 °C, respectively (Fig.6). Simultaneously nitrate ions might come from indirect oxidation of HOCl and the hydroxyl radical. According to the results of research, Cu/Zn was more suitable for the oxidation of ammonium. The highest formation of nitrate was also found with Cu/Zn cathode and decreased finally with the time according to the standard discharge.
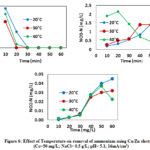 |
Figure 6: Effect of Temperature on removal of ammonium using Cu/Zn electrode |
Treatment of Real Wastewater Under Optimal Conditions
In this experiment, we used the influent of Tsinghua University Wastewater Treatment Plant, which consisted of domestic sewage wastewater from region of Beijing, without any pretreatment. The COD concentration of the raw sewage was 300 mg/L and the NH4+ – N concentration was 90 mg/L (Table 1).
Table 1: Characteristics of real wastewater used
| Parameter | Unit | Value |
| pH | – | 7.75 |
| Chemical oxygen demand (COD | mg/L | 300 |
| NH4+ -N | mg/L | 90 |
| PO43- | mg /L | 5.5 |
The alloy electrode of Cu/Zn with a current density of 18.32 mA/cm2, a space size between the two electrodes of 1.1 cm, temperature of 40 °C and an added amount of 0.5 g/L of NaCl, the raw sewage was electrolyzed, and the results are shown in (Fig 7). The electrodes have an excellent effect on the removal rates of COD and NH4+ – N from the raw sewage. After 30 min of electrochemical reaction under the optimal conditions, the COD in raw sewage was 185 mg/L, a removal rate of 38%; and the total NH4+ – N and PO43- concentration have removed.
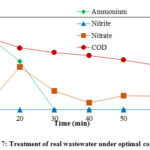 |
Figure 7: Treatment of real wastewater under optimal condition |
Comparison Among the Electrodes
The Cu/Zn electrode is the most effective of all three electrodes used subsequently followed by the Fe electrode and finally the Cu electrode. The respective values of these three electrodes for reduction of ammonium without adding NaCl is 99.8%, 97.8% and 94.4% (Fig 8). The process of oxidation of ammonium is a complex phenomenon. Conversion the ammonium to nitrite or nitrate is a reductive reaction. It must be recognized that conversion was mainly influenced by these electrodes using a cathode.
Nevertheless results reveal that the Cu/Zn electrode has a higher yield for the reduction of ammonium because it has more effective properties in electro-catalytic but also has high over potential for hydrogen evolution in vicinity of cathode.
According to the result iron electrode is also very effective due its greater electron donating especially at low pH. In our experiment, the pH of the solution before treatment was 5.6, whereas at high pH we found the phenomenon of passivation to the electrode surface area, which limited the oxidation process of ammonium.
Previous studied revealed Cu/Zn electrode comparing to copper electrode was higher over potential. It seems that Cu electrode with more negative electrode inhibit the amount of hydrogen produced and finally limit the rate oxidation of ammonium.
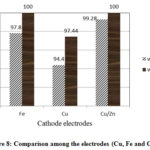 |
Figure 8: Comparison among the electrodes (Cu, Fe and Cu/Zn) |
Degradation kinetic of NH4+ – N
The degradation kinetic of NH4+ was studied to examine the order of the reaction. Despite its complexity and it is being controlled by many interacting variables, we attempt to throw some light on the kinetics of the present process. It was assumed that the process takes place according to first order mechanism, which can be expressed by:
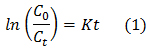
Where;
Co is the initial concentration (mg/L), Ct is the concentration at time t (mg/L), K is the rate constant (min.-1) and t is the time (min.).
Figures 9 show the effect of current density (16 mA/cm2), temperature (20°C, 30°C, and 40°C), 0.5 g/L of NaCl, pH value and initial concentration of ammonium (50mg N/L) on the kinetics of the reaction with Iron electrode as cathode. The present data agree with equation 1 (Eq.1), however, two (2) slopes are differentiated indicating the presence of two rates of removal. In first stage which occurs during few minutes, the rate is higher than the rate in second stage. The fast rate of removal in the first few minutes is attributed to the flocs which are still free so the adsorption rate is very fast. The low rate of removal in second stage is due to the fact that the flocs was reduced (the number of free flocs is few) so the adsorption rate becomes very slow. The presence of soluble iron in the solution and a high area of the working electrode iron facilitate the removal of NH4+ – N and denitrification. The difference between the electrode Cu and Fe electrode for ammonium removal is due to the number of electron giving.
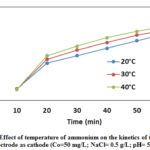 |
Figure 9: Effect of temperature of ammonium on the kinetics |
Mechanism and Reactions
In the electrolytic, ammonium was eliminated with anodic electro-oxidation. Electro-oxidation of ammonium occurred in two ways:
Ammonium was oxidized directly to the surface of the anode through the electrode such as Ti/IrO2-Pt.
Indirectly with the assistance of a mediator oxidation such as active chlorine Cl2, HOCl, or OCl– which were produced at the anode (Ghafari et al., 2008 , Mouedhen et al. 2008). In indirect oxidation, chloride in the solution was oxidized to chlorine at the anode, and then the chlorine immediately reacted with H2O to form hypochlorite (HOCl) (Panizza and Cerisola, 2009):
Ammonium was oxidized directly to the surface of the anode through the electrode such as Ti/IrO2-Pt.
Indirectly with the assistance of a mediator oxidation such as active chlorine Cl2, HOCl, or OCl– which were produced at the anode (Ghafari et al., 2008 , Mouedhen et al. 2008). In indirect oxidation, chloride in the solution was oxidized to chlorine at the anode, and then the chlorine immediately reacted with H2O to form hypochlorite (HOCl) (Panizza and Cerisola, 2009):
2Cl– → Cl2 + 2e–
Cl2 + H2 O → HOCl + H+ + Cl–
Finally, ammonium was oxidized to nitrogen gas or nitrate through the electrochemically oxidants such as hypochlorus acid and hypochlorite ion in the vicinity of the anode (Reyter et al., 2010; Vlyssides et al., 2002):
3NH+4 + 3HClO → N2+ 3H2O + 5H+ + Cl–
NH4+ + 4HOCl → NO3– + 6H+ + 4Cl– + H2
Reduction
NO3– → NO2– → N2
The reducing environment in the electrochemical cell was influenced by the electrode material.
Conclusion
Electrochemical method for ammonium removal with Cu, Fe and Cu/Zn as cathode and Ti/IrO2 as anode was considered as a good method. It appears that through the experiment Cu/Zn electrode as cathode is better than Fe and Cu electrode for the ammonium removal with NaCl or without NaCl. The high formation rate of nitrate without NaCl was found with Cu/Zn and with NaCl addition it was Cu electrode as cathode .With the increase of the temperature, removal rate of ammonium improved on time specially with Cu/Zn electrode around 30 °C whereas the highest formation of nitrate was found with Cu around 30 °C. Electrochemical degradation of ammonium with the concentration of ammonium was studied follow the first-order model law. The kinetic study showed that current density (current passed used during experience per unit electrode area) is an appropriate design
Acknowledgements
The present study was funded by the 863 Program (2007AA06A410) of the Chinese Ministry of Science and Technology.
References
- APHA, (1998). Standard Methods for Examination of Water and Wastewater, American Public Health Association, Washington, , p. 87.
- Arroyo M. G., V. P.-Herranz, M. T. Montanes, J. Garcia- Anton, and J. L.. Guinon, (2009), “Effect of pH and chloride concentration on the removal of hexavalent chromium in a batch electrocoagulation reactor”, J. Hazard. Mater., 169 (1-3), 1127-1133.
CrossRef - Chiang L. C., Chang J. E., Wen, T. C., (1995) Indirect oxidation effect in electrochemical oxidation treatment of landfill leachate. Water Research, ,29(2):671-678
CrossRef - Deng Y, Englehardt J D. (2007). Electrochemical oxidation for landfill leachate treatment [J]. Waste Management, 27(3): 380-388.
CrossRef - Ghafari S., M. Hasan, M. Aroua, (2008). Bio-electrochemical removal of nitrate from water and wastewater – a review, Bioresour. Technol. 99 3965–3974
CrossRef - Gupta S. K., Gupta A. B., Gupta R. C., Seth A. K., Bassain J. K., Gupta A.. (2000). Recurrent acute respiratory tract infections in areas with high nitrate concentrations in drinking water. Environmental Health Perspectives, ,108:363–366.
CrossRef - Kim K.W., Y.J. Kin, I.T. Kim, G. Park, E. Lee, (2006) Electrochemical conversion characteristics of ammonia to nitrogen, Water Res. 40 1431–1441.
CrossRef - Kim T.H, Park C, Shin E.B. (2002) Decolorization of disperse and reactive dyes by continuous electrocoagulation process. J. Desalination, 150(2):165-175
CrossRef - Lee J.-K. et al., (2002) Residual Chlorine Distribution and Disinfiction during Electrochemical Removal of Dilute Ammonia from an Aqueous Solution, J. Chem. Eng. Jpn., 35, 285.
CrossRef - Li L., Liu Y. (2009) Ammonia removal in electrochemical oxidation: mechanism and pseudo-kinetics, J. Hazard. Mater. 161:1010-1016
CrossRef - Lin S.H, Wu C.L. (1996) Electrochemical removal of nitrite and ammonia for aquature. Water Res., 30(3):715-721
CrossRef - Martin G. (1979). The problem of nitrogen in the water. Techniques and documentation, , 657-659
- Mouedhen, G., Feki, M., Wery, M.D.P. and Ayedi, H. (2008) Behavior of aluminum electrodes in electrocoagulation process. Journal of Hazardous Materials 150(1), 124-135.
CrossRef - Nouri J., A.H.Mahvi, and E.Bazrafshan,” Application of electrocoagulation process in removal of Zinc and Copper from Aqueous solutions by aluminum electrodes”, Int. J. Environ., 4 (2), (2010), 201-208.
- Pérez G., J. Saiz, R. Ibáñez, A.M. Urtiaga, I. Ortiz, (2012a). Assessment of the formation of oxidation by-products during the electrocatalytic treatment of ammonium from landfill leachates, Water Res. 46 2579–2590.
CrossRef - Pérez G., R. Ibáñez, A.M. Urtiaga, I. Ortiz (2012b). Kinetic study of the simultaneous electrochemical removal of aqueous nitrogen compounds using BDD electrodes. Chemical Engineering Journal 197 475–482
CrossRef - Rajic Lj, D. Berroa, S.Gregor, S. Elbakri, M.MacNeil, A. N. Alshawabkeh (2017). Electrochemically-induced Reduction of Nitrate in Aqueous Solution. Int. J. Electrochem. Sci., 125998 – 6009,
CrossRef - Reyter D., D. Bélanger, L. Roué, Nitrate removal by a paired electrolysis on copper and Ti/IrO2 copled electrodes – influence of the anode/cathode surface area ratio, Water Res. 44 (2010) 1918–1926.
CrossRef - Shrimali M, Singh K P. New methods of nitrate removal from water[J]. Environmental pollution, 2001, 112(3): 351-359.
CrossRef - Vlyssides A.G., P.K. Karlis, N. Rori, A.A. Zorpas, Electrochemical treatment in relation to pH of domestic wastewater using Ti/Pt electrodes, J. Hazard. Mater. 95 (2002) 215–226.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









