A Comparative Study of Iron Removal Efficiency by Precipitation Method and Deferrization Unit of DAN DADJI-Illéla Drilling.
Yahouza Zaneidou, Manzola Abdou Salam*, Amadou Haoua and Laouali Mahaman Sani
Chemistry Department, Faculty of Science and Technology, Abdou Moumouni University of Niamey BP. 10662 Niamey - Niger
Corresponding Author E-mail: abdoussalam_manzola@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/360108
Article Received on : 02 Dec 2019
Article Accepted on : 03 Jan 2020
Article Published : 20 Jan 2020
The paper is a comparative study on deferrization efficiency of two methods on the waters of rural drilling of Dan Daji (Niger) which have high iron concentration (1.69 mg/L): the deferrization by oxidation-precipitation method and the deferrization made by a modern unit of deferrization. Before comparison, a synthetic solution was prepared by adding a certain amount of FeSO4.7H2O in bicarbonate aqueous solutions and studied. The natural water was characterized by physico-chemical analyzes. The two methods are appropriate for the deferrization of synthetic solutions and Dan Daji Illela iron poisoning field waters. The oxidation-precipitation method can be used as a pretreatment method before the deferrization unit. DRX analyzes of the precipitates obtained showed the coexistence of two (2) solid phases; goethite (α-FeOOH) G and lepidocrocite (-FeOOH) L for [HCO3-] = 488 mg/L; pHi = 8; [Fe2+]0 = 5 mg/L and T = 30 °C. For the other iron and bicarbonate concentrations and for the other pHi studied, the precipitating phase was mainly goethite.
KEYWORDS:Carbonate Medium ; Deferrization Unit ; Groundwater ; Initial Ph ; Iron Oxyhydroxide ; Precipitation.
Download this article as:| Copy the following to cite this article: Zaneidou Y, Salam M. A, Haoua A , Sani L. M. A Comparative Study of Iron Removal Efficiency by Precipitation Method and Deferrization Unit of DAN DADJI-Illéla Drilling. Orient J Chem 2020;36(1) |
| Copy the following to cite this URL: Zaneidou Y, Salam M. A, Haoua A , Sani L. M. A Comparative Study of Iron Removal Efficiency by Precipitation Method and Deferrization Unit of DAN DADJI-Illéla Drilling. Orient J Chem 2020;36(1). Available from: https://bit.ly/2TGt1o2 |
Introduction
With an ever-increasing population and increasing demographic pressure, Niger faces surface water scarcity as in most arid climate developing countries. To meet the water needs of the population, the Niger Republic government has exploited deepwater aquifers, the only sources of water supply for the population. The increasing demands of water make their exploitation vital for the development of these countries. Since 1996, more than 6207 boreholes and 10005 wells have been drilled. These boreholes play a crucial role in providing water to the population. A study carried out by the Ministry of Hydraulics, Environment and Desertification Control (MHE/LD) in 2005 on the physico-chemical analyzes of water showed that, out of a total of 148 boreholes studied, 28.4 % have an iron concentration well above 0.3 mg/L (value recommended by WHO). Analyzes by region shows that the percentage of drilling in the regions with iron concentrations above 0.3 mg/L varies from 16 to 70 %: 20 % in Diffa region (Bosso 2.34 mg/L), 16 % in Dosso region (Rountoua 2.02 mg/L), 27 % in Maradi region (Halbawa 7.6 mg/L) and 70 % in Tahoua region (Kagarki kagarkawa 8.2 mg/L; Edir 5.35 mg/L; Bagaroua 4.7 mg/L; Hayayé 4.75 mg/L). The highest concentrations were recorded in Tahoua and Maradi regions with 8.2 and 7.6 mg/L respectively. An overload of iron in the human body can lead to primary hemochromatosis (poor regulation of iron absorption by the intestine) and even liver cancer (risk of liver cancer), these disorders generally occur when the concentration of iron in water is greater than 10 mg/L1. At concentrations above 0.3 mg/L, the presence of iron in water affects the organoleptic qualities of the water (unpleasant taste, smell, and color) and is responsible over time for corrosive deposits in pipes. These formed deposits can be the site of highly toxic bacterial colonies.3,4,5,6,7,8,9,10,11 In the absence of other resources, groundwater, reserve in Niger, is the only alternative to face this shortage. The exploitation of these groundwater tables is necessary to cover a large part of the enormous water needs of the populations. Unfortunately, most of the groundwater in the Tahoua region has a high content of iron (II) and manganese, leading to insoluble and encrusted deposits of iron oxyhydroxides when these waters are in contact with oxygen in the air. Some of the hydroxides settle as silt and give a rusty color and unpleasant taste to the water.12 Indeed, once in contact with oxygen in the air, iron (II) oxidizes to iron (III) and precipitates as Fe(OH)3 and probably oxyhydroxides of iron (III). At lower concentrations, the problems associated with the presence of iron in drinking water are aesthetic.18,19
The presence of iron in water leads to a certain speciation which corresponds to its distribution between the different physico-chemical species, thus defining the different physico-chemical forms present and their relative evaluation. This speciation is based on the behavior of iron in aqueous solution, it depends on several factors such as pH, initial concentration, temperature and ageing time of the solution, etc. The influence of pH is directly related to the dissociation equilibria that partly govern the distribution of the different species of iron (II) and iron (III) that can be formed in the solution. These species are generally Fe(OH)3, Fe(OH)2+, Fe(OH)4– for iron(III) and Fe(H2O)62+, FeCO3, Fe(OH)2, FeOH+ for iron (II)20,21. The predominance of these species in a solution is related to the stability constants of the iron(II) and Fe(III) complexes, these different constants are recorded in table I22. In view of this worrying situation, it is imperative to take measures to improve the quality of drinking water in rural areas. Currently several methods of iron removal are being developed. These processes include, among others, physico-chemical processes based on the use of stronger or weaker oxidants (chlorine, potassium permanganate, oxygen and ozone), biological processes involving micro-organisms, catalytic processes based on adsorption and oxidation on the surface of a specific material and membrane processes2,7,23,24. These treatment methods are not without limits in the implementation, both in the use of certain materials and in the control of certain physico-chemical parameters and especially in the accessibility of the necessary materials and chemicals. Among all these methods we have retained the elimination of iron by oxidation precipitation with oxygen from the air in the carbonate medium, in the form of oxyhydroxides (lepidocrocite), which is a method closer to reality and easily accessible.4
The objective of the present study was to investigate the performance of the deferrization and precipitation deferrization unit as a feasible and suitable deferrization method. Deferrization studies were carried out under various equilibrating conditions like the effect of initial pH, bicarbonate concentration and iron (II) concentration. Detailed precisions during the deferrization mechanism and the kinetic studies are presented. The precipitated products were characterized by DRX diffraction and FTIR Fourrier Transform Spectroscopy.
Materials and Methods
In our study for precipitation fertilization, we used an assembly consisting of a 1-litre beaker and a mechanical agitator. All manipulations were carried out in the beaker under mechanical agitation at atmospheric pressure and 30°C in order to increase the contact between the oxygen in the air and the Fe2+ ions in the solution, which is the driving principle behind the evolution of the system leading to the oxidation of the Fe2+ ions and the precipitation of Fe(OH)3. Iron solutions were prepared by introducing variable amounts of FeSO4.7H2O iron sulfate heptahydrate into bicarbonated water. The bicarbonated water was prepared by dissolving varying amounts of NaHCO3 in distilled water. The amount of iron was introduced after setting a desired initial pH value by splashing CO2. The stopwatch and the stirrer are triggered at the same time for a duration of 60 minutes, which corresponds to the time set for all the experiments. Agitation introduces atmospheric oxygen into the solution. Contact of the solution with atmospheric air causes the release of dissolved CO2, which leads to alkalinization of the medium, resulting in an increase in pH. This increase in pH favours the oxidation of Fe2+ to Fe3+ and the precipitation of Fe(OH)312. The overall precipitation response is as follows:
Fe2+(aq) + 2HCO3–(aq) + 1/4O2(g) + 1/2H2O(l) ↔ Fe(OH)3(s) + 2CO2 ↑ (R1)
The iron hydroxide obtained according to the R1 reaction is very unstable, which explains its evolution towards another iron oxyhydroxide more thermodynamically stable, it is lepidocrocite in its non-hydrated form according to the following reaction:
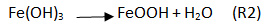
Several series of tests were performed by varying: the concentration of iron, bicarbonate and pHinitial. Iron solutions were prepared by introducing variable amounts of FeSO4.7H2O iron sulfate heptahydrate into bicarbonated water. Three (3) iron concentrations were studied in our study: 5 mg/L, 10 mg/L and 15 mg/L by dissolving 24.82 mg; 49.64 mg and 74.46 mg of FeSO4.7H2O respectively in a 1-litre vial. The bicarbonated water was prepared by dissolving varying amounts of NaHCO3 in distilled water. Three (3) bicarbonate solutions of different concentrations were prepared: 122 mg/L, 244 mg/L and 488 mg/L. The effect of iron concentration, bicarbonate concentration and initial pH was studied. The initial pH values were determined by splashing CO2 into the bicarbonated water. For our experiment, the initial pH values studied are: pHi 6, pHi 7 and pHi 8. To determine the concentration of iron in solution during the precipitation experiment, 1 ml samples were taken using a 5 ml syringe with a 0.45 µm membrane filtration system. Samples of 1mL were taken every 2 minutes, then every 10 minutes of precipitation. To the 1 mL sampled, we successively added 1mL hydroxylamine, 2 ml ammonium acetate and 2 mL orthophenanthroline. After introducing these reagents, the mixture was completed to 50 mL with distilled water in a 50 mL vial. Iron levels were analyzed using a Zuzi Spectrophotometer Model 4101 spectrophotometer at a wavelength of 510 nm. The pH was measured using a pH meter marked VWR pHenomenal pH 1100 H. The obtained solids were filtered through a 0.45 µm filter, then characterized by x-ray diffraction (XRD). XRD was carried out at room temperature with a Philips X’ PERT PRO diffractometer in step scanning mode using Cu_Kα radiation. The XRD patterns were recorded in the scanning range 2θ = 15 – 60°. A small angular step of 2θ = 0.017° and a fixed counting time of 4 s were used.
Results and Discussion
Precipitation in Aqueous Solutions with [HCO3–] = 0 mg/L
The 1.0 L of free bicarbonate aqueous solution consisting of deionized water, with an initial pH of 5.87 was introduced into the cell. An amount of FeSO4.7H2O is added to the solution and the experiment is started. Iron (II) concentration and pH are recorded. Figures 1a and 1b show iron (II) concentration and pH evolution versus time.
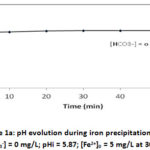 |
Figure 1a: pH evolution during iron precipitation . |
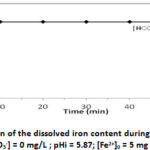 |
Figure 1b: Evolution of the dissolved iron content during iron precipitation for |
Figure 1a shows an abrupt drop of pH immediately when Iron (II) is added but the iron (II) concentration remains constant at 5 mg/L (figure 1b). The reached pH is 4.66 within a minute. The drop of pH is accompanying by H+ liberation according to the reaction:
![]()
Consequently there is not precipitation with deionized water when bicarbonate concentration [HCO3-] = 0 mg/L. In the aim of massive and fast precipitation, bicarbonate solutions were used.
Precipitation Experiments – Synthetic Solutions
Synthetic Solutions – Influence of HCO3– Concentration
To study the influence of the presence of bicarbonate in the precipitation medium and the effect of its concentration, we conducted 4 tests at different concentrations : [HCO3–] = 122 mg/L; 244 mg/L and 488 mg/L for pHi = 8; [Fe2+]0 = 5 mg/L and at 30°C. The results are shown in Figures 2a and 2b.
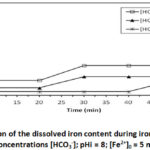 |
Figure 2a: Evolution of the dissolved iron content during iron precipitation |
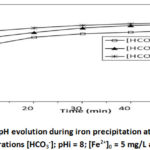 |
Figure 2b: pH evolution during iron precipitation at different concentrations |
The results presented in Figure 2a shows the influence of the presence of bicarbonate and the effect of its concentration. This presence causes a sudden drop in iron concentration, and the higher the bicarbonate concentration, the higher the drop. Thus in less than 2 minutes, for a concentration of [HCO3–] = 122 mg/L the iron content drops to [Fe2+] = 0.97 mg/L or an 80 % elimination, for [HCO3–] = 244 mg/L it drops to [Fe2+] = 0.485 mg/L or an elimination of 90 %, and finally for [HCO3–] = 488 mg/L the iron content drops to [Fe2+] = 0.242 mg/L or an elimination of 97%. The HCO3– concentration 488 mg/L and initial pH 8 could bring down the level of Fe2+ within the tolerance limit, [Fe2+] = 0.242 mg/L (WHO guideline value = 0.3 mg/L). Iron precipitation is immediately followed by a drop in pH (Figure 2b). The higher the concentration of bicarbonate increases, the poorer the drop. The largest drop is observed for [HCO3–] = 122 mg/L, where the pH has reached 6.656 for an initial pH of 8. bicarbonate ions make the medium slightly alkaline, favorable not only to the oxidation of Fe2+ but also to the precipitation of certain iron oxyhydroxides such as lepidocrocite12,13,14,15,16,17. The oxidation reaction of Fe2+ iron in the presence of oxygen without bicarbonate leads to the formation of Fe3+ ferric ions, as indicated in the equation of the following reaction:
4Fe2+ + O2 + 2H2O → 4Fe3+ + 4OH– (R3)
The ferric iron thus formed, after undergoing hydrolysis mechanisms, precipitates as ferric hydroxide Fe (OH)3 according to the following precipitation reaction:
4Fe3+ + 4OH– + 8H2O → 4Fe(OH)3 + 8H+ (R4)
The combination of R3 and R4 gives the overall reaction of the precipitation:
4Fe2+ + O2 + 10H2O → 4Fe(OH)3 + 8H+ (R5)
The H+ protons released during the R5 reaction acidify the medium, preventing iron precipitation. On the other hand, in the bicarbonate medium, these protons will react with the bicarbonate ions HCO3– (R1) and maintain the basic medium causing a rapid and massive precipitation of iron oxyhydroxides, confirming the results obtained by Wided et al.12. From the 25 th minute onwards, iron oxide was redissolved according to the R6 reaction, leading to an increase in Fe2+ concentration and an increase in pH (Figure 2b). The highest redissolution was observed for [HCO3–] = 122 mg/L where the iron content has increased to [Fe2+] = 1.941 mg/L, probably due to a high production of H+ protons on the one hand, and a low consumption of H+ ions by the bicarbonate ion on the other hand.
 |
Figure 2c: a, b and c. X-ray diffractograms |
The solids formed during these experiments were filtered through a 0.45 μm membrane, dried at room temperature and then analyzed by X-ray diffraction (XRD). The diffractograms obtained for different bicarbonate concentrations, at [Fe2+] = 5 mg/L; pHi = 8 and T= 30 °C are shown in Figure 2c. The analyzes of these diffractograms shows the coexistence of two (2) varieties of crystallized iron oxyhydroxides for [HCO3–] = 488 mg/L: goethite (α-FeOOH) noted G and lepidocrocite (g-FeOOH) noted L25,26,27. For other lower concentrations, lepidocrocite disappears (Figure 2c). Conflicting results have been observed by other authors. Music et al.11 and Legrand et al.12,18 discovered on their part that the presence of bicarbonate in solution prevents the formation of lepidocrocite. These authors explained that bicarbonate ions can incorporate into the precipitation structure and inhibit their crystallization, resulting in an amorphous end product.
Synthetic Solutions – Influence of Initial pH
To study the effect of pHi we performed 4 tests at different pHi: 6; 7 and 8 for [HCO3–] = 488 mg/L, [Fe2+]0 = 5mg/L at T = 30 °C. The results are shown in Figures 3a and 3b.
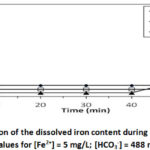 |
Figure 3a: Evolution of the dissolved iron content during iron precipitation |
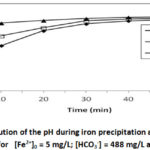 |
Figure 3b: Evolution of the pH during iron precipitation |
Figure 3a shows the evolution curves of the iron(II) concentration as a function of time for different pHi. Examination of these curves shows that the higher the pHi increases, the more massive the precipitation of iron. Indeed, at pHi = 8, more than 95.16 % of the ferrous iron was removed for a precipitation time of less than 2 minutes, the iron content drops to [Fe2+] = 0.242 mg/L, confirming Catherine Lessard’s work in 199923, which showed that iron oxidation by oxygen is much more important and instantaneous when the pH is around 8. This precipitation leads to the instant formation of Fe(OH)3 at around pH 812,23. For pHi = 7 the iron content drops to [Fe2+] = 0.485 mg/L or 90.40 % elimination and for pHi = 6 the iron content drops to [Fe2+] = 0.73 mg/L or 85 % elimination. These results clearly show that the oxidation-precipitation process of ferrous iron by oxygen from the air is also important even in bicarbonate media with a pHi of 6 or less. From the results presented in Figure 3a we also note that the oxidation-precipitation phenomenon is instantaneous at all the pHi studied, unlike the results obtained by12 which shows that the oxidation-precipitation process is instantaneous only at pHi 8. This can be explained from the Fe2+ concentration used in our tests, which is in the order of 5 mg/L instead of 25 mg/L used by12. From the 40th minute onwards, iron oxide is redissolved according to the R6 reaction, resulting in an increase in Fe2+ concentration and an increase in pH (Figure 3b) only for pHi = 8. This redissolution resulted in an increase in iron content to [Fe2+] = 0.97 mg/L, less significant than that obtained for [HCO3–] = 122 mg/L ([Fe2+] = 1.941 mg/L) at the same pHi = 810. The solids formed during these experiments were filtered through a 0.45 μm membrane, dried at room temperature and then analyzed by X-ray diffraction (XRD). The diffractograms obtained for the different pHi at [Fe2+] = 5 mg/L; [HCO3–] = 488 mg/L and T= 30 °C are shown in Figure 3c.
The analysis of these diffractograms shows the coexistence of two (2) varieties of crystallized iron oxyhydroxides for pHi = 8: goethite (α-FeOOH) noted G and lepidocrocite (g-FeOOH) noted L26. For the other lower pHi, lepidocrocite disappears (Figure 2c). As pHi decreases, goethite crystallizes more and more. Conflicting results have been observed by other authors. Indeed, Wided, 201712 found that precipitation of iron in a bicarbonate medium of high concentration and pH = 8 only promotes the formation of an amorphous iron oxyhydroxide (ferrihydrite). So the precipitation of iron in the neutral bicarbonate medium promotes the production of goethite. Figure 3c also shows that the diffractogram obtained at pHi = 6 is almost identical to that of pure goethite, as reported in the literature21,25. These results are contradictory to those obtained by12 or at pHi = 6 and 7, lepidocrocite is formed.
 |
Figure 3c: a, b and c. X-ray diffractograms Click here to View Figure |
Synthetic Solutions – Influence Of The Initial Fe2+ Concentration
To study the effect of iron content we conducted 4 tests at different concentrations: 5 mg/L; 10 mg/L and 15 mg/L for [HCO3–] = 488 mg/L, pHi = 8 and at a temperature of 30°C. The results are shown in Figures 4a and 4b.
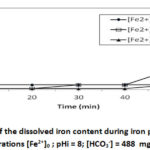 |
Figure 4a: Evolution of the dissolved iron content during iron precipitation. |
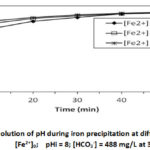 |
Figure 4b: Evolution of pH during iron precipitation at different concentrations |
The results presented in Figure 4a show instantaneous precipitation at pHi = 8, for all the different initial iron concentrations studied. After about 2 minutes of precipitation, the iron content drops to 0.2 mg/L and stabilizes until the 25th minute for [Fe2+]0 = 10 mg/L, after which time there is an increase in the iron content probably due to a redissolution of the precipitated product. This stabilization continues until the 40th minute for [Fe2+]0 = 5 mg/L, after which time there is an increase in iron content as before, resulting in an iron removal rate of 96 % for each situation. For the initial iron concentration [Fe2+]0 = 15mg/L, the iron content drops to 0.98 mg/L (elimination rate 80.40 %), stabilizes until the 40th minute, at which time there is an increase in iron content to [Fe2+] = 3.88 mg/L. These results indicate that iron removal is more important when the iron concentration is low. This can be explained from the oxidation-precipitation mechanism, during which protons are released according to the amount of Fe (II) initially introduced into the solution. We find that the higher the initial Fe(II) content, the higher the production of H+ ions (Figure 4b), leading to a redissolution of precipitates in the immediate vicinity of these protons according to equation (R6). These results allow us to affirm that, the higher the iron content, the greater the redissolution of Fe(OH)3 is according to the reaction equation R6.
Fe(OH)3(s) + 3H+(aq) → Fe2+ + 3H2O (R6)
 |
Figure 4c: a, b and c. X-ray diffractograms |
The solids formed during these experiments were filtered through a 0.45 μm membrane, dried at room temperature and then analyzed by X-ray diffraction (XRD). The diffractograms obtained for the different initial concentrations of iron pHi = 8; [HCO3–] = 488 mg/L and T= 30 °C are shown in Figure 4c. The analysis of these diffractograms shows the coexistence of two (2) varieties of crystallized iron oxyhydroxides for [Fe2+]0 = 5 mg/L : goethite (α-FeOOH) noted G and lepidocrocite (-FeOOH) noted L27. For the other initial iron concentrations of 10 mg/L and 15 mg/L the diffractograms are identical. These diffractograms are also identical to the diffractogram obtained for [HCO3–] = 488 mg/L; [Fe2+]0 = 5 mg/L; pHi = 7 and T = 30°C which states that the precipitation of iron in the neutral bicarbonate medium promotes the production of goethite.
Precipitation Experiments – Raw Water of the DAN DAJI – ILLELA Drilling – Effect of HCO3– Concentration.
In the second series of experiments the efficiency of oxidation deferrization and deferrization by the DAN DADJI deferrization unit – Illéla was tested with natural water from the DAN DADJI drilling – Illéla under the same conditions as those of synthetic solutions. The physico-chemical characteristics of the drilling water before and after the DAN DADJI – Illéla deferrization unit are shown in table I:
Table 1: The results of the analysis of the physico-chemical parameters before and after the deferrizer
| Physico-chemical parameters (mg/L) |
Before deferrizer |
After deferrizer |
| pH | 7.35 | 7.25 |
| CE (µS/cm) |
212 | 209 |
| T (°C) |
26.5 | 26.1 |
| SO42- | 36 | 37 |
| F– | 0.03 | 0.33 |
| HCO3– | 37.5 | 37.5 |
| NO3– | 1.32 | 1.76 |
| NO2– | 0.0099 | 0.0099 |
| Cl– | 1 | 1 |
| Fe2+ | 1.69 | 0.136 |
| Na+ | 7049 | 7.43 |
| Ca2+ | 12 | 12 |
| Mg2+ | 4.35 | 4.35 |
| K+ | 15.93 | 15 |
| Mn2+ | 1.2 | 1.2 |
The results in Table I show that the physical parameters (pH, electrical conductivity and temperature) and most chemical parameters (SO42-, F–, HCO3–, NO3–, NO2–, Cl–, Mg2+, K+ , Na+, Ca2+ and Mn2+) are practically unchanged, before and after the deferrizer, only the Fe2+ content evolved from [Fe2+] = 1.69 mg/L to 0.136 mg/L, i.e. an iron removal rate of 91.95 %, confirming the efficiency of the deferrization unit. Consequently, the deferrization unit of DAN DAJI – ILLELA could bring down the level of Fe2+ within the tolerance limit, [Fe2+] = 0.136 mg/L (WHO guideline value = 0.3 mg/L).Indeed, currently the water from the DAN DAJI – ILLELA borehole is treated using a deferrization unit installed in ILLELA. The deferrizer constantly breaks down, causing recurrent shortages of drinking water. For alternative solutions a series of deferrization tests by the precipitation method was carried out on these waters by varying the quantity of bicarbonate under the same conditions as the synthetic solutions. The different concentrations studied are: HCO3–] = 37.5 mg/L ; 122 mg/L ; 244 mg/L and 488mg/L for pHi = 7.35, [ Fe2+]0 = 1.69 mg/L and at 30°C. The results are shown in Figures 5a and 5b.
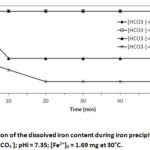 |
Figure 5a: Evolution of the dissolved iron content during iron precipitation |
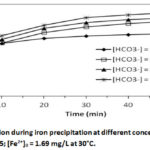 |
Figure 5b: pH evolution during iron precipitation |
Figure 5a shows that the precipitation of Fe2+ iron in the stirred carbonate medium is instantaneous and massive as the concentration of bicarbonate increases. The same results were observed for synthetic solutions. For [HCO3–] = 37.5 mg/L, precipitation is very low or even non-existent, the iron concentration [Fe2+] = 1.69 mg/L remains constant throughout the operation. For [HCO3–] = 244 mg/L, precipitation is instantaneous and after one minute of precipitation, the iron concentration drops to [Fe2+] = 1.45 mg/L. After 10 minutes of precipitation, the iron concentration drops to 0.72 mg/L and remains constant throughout the operation. For [HCO3–] = 488 mg/L, precipitation is instantaneous and after one minute of precipitation, the iron concentration drops to [Fe2+] = 0.72 mg/L and after 20 minutes of precipitation, the iron concentration drops to 0.24 mg/L or 89.8 0% iron removal, and remains constant until the end of the experiment. These results can be explained from the speciation of Fe(II) in aqueous solution. Indeed, the study of the speciation of Fe(II) in the bicarbonate medium shows that several Fe(II) complexes can be formed, namely FeCO3, Fe(OH)2, [Fe(CO3)2]2-, FeHCO3, Fe(OH)+, Fe(CO3)OH–, [Fe(HCO3)]+20,28,29,30,31. Some of these complexes contribute to retaining iron in solution. On the other hand, other complexes contribute to a very high precipitation rate of Fe (II) at certain concentrations of NaHCO322. Figure 5b confirms the increase in pH during iron precipitation as in the case of synthetic solutions. These results corroborate many works.12,22
Conclusion
From the present results, we can conclude as follows :
Precipitation method and deferrization unit are appropriate for the deferrization for synthetic solutions and Dan Daji Illela iron poisoning field waters.
There is no important precipitation with free bicarbonate deionized water.
The precipitation method shows higher iron precipitation capacity for deferrization for synthetic water in high bicarbonate concentration and high initial pH.
The precipitation method shows equal iron precipitation capacity for deferrization for Dan Daji Illela iron poisoning field waters as compared to deferrization unit.
The iron removal capacity of precipitation method decreases with a raise in iron (II) concentration contrary to the initial pH increases for synthetic solution.
Acknowlegment
The authors thank especially to the Laboratoire de Valorisation des Matériaux utiles, Centre National de Recherche en Sciences
des Matériaux, Technopole de Borj Cedria, Tunisie for recording DRX.
Conflicts of interests
The authors declare that they have no conflict of interest.
References
- Michael, C. K. Liver Cancer. 2014, 3(1), 31- 40.
CrossRef - Amadou, H. ; Mahaman, S. L. ; Manzola, A. S. J. App. Bio. 2014, 80, 7161 – 7172.
CrossRef - Boubakar, H. A. Doctoral thesis U.A.M. 2010, 249-260.
- Dehou, S. C. Doctoral thesis Univ. Lille. 2011, 103 – 143.
- Ruiti, M.; Bechir, B. T. In. J. of Innov. App. Stu. 2015, 10, 694-700.
- Donald, E. l. M. Sc. 1998, 220-229.
- OMS. Guidelines for drink water quality. 2011, 4th edi., 541-545.
- OMS. Guidelines for drink water quality. 2017, 4th edi., 564-569.
- Christelle, N. M. Sc. 2006, 151-159.
- Anne-Sophie, S. Doctoral thesis Univ. Lorraine. 2012, 255-261.
- Vong, L. Doctoral thesis Univ. Aix-Marseille. 2008, 171-188.
- Wided, M. Doctoral thesis Univ. Cathage. 2017, 60-77.
- Millero, F. J.; Sotolongo, S.; Izaguirre, M. Geochim. Cosmochim. Acta. 1987, 51, 793-801.
CrossRef - Schwertmann, U.; Cambier, P.; Murad, E. Clays Clay Miner. 1985, 33, 369-378.
CrossRef - Misawa, T.; Hashimoto, K.; Shimodaira, S. Corr. Sci. 1974, 14, 131-149.
CrossRef - Schwertmann, U.; Fechter, H. Clay Miner. 1994, 29, 87-92.
CrossRef - Gu, X. Y.; Hsu, P. H. Soil Sci. Soc. Am. J. 1987, 51, 469-474.
CrossRef - Michaël, D.; Catherine, B.; Florence, M.; Sébastien, S.; Joachim, S.; Pierpaolo, Z. Bull. Soc. géol. 2002, 173(3), 265-270.
- Legrand, L.; Savoye, S.; Chausse, A.; Messina, R. Elec. Acta. 2000, 46, 111-117.
CrossRef - Magdalena, S. J.; Melchor G. L. V.; Frank, J. M. M. Chem. 2004, 85, 27– 40.
- Schwertmann, U.; Thalmann, H. Clay min. 1976, 11,189-198.
CrossRef - Wang, Z.; Xu C.; Cao, X.; Xu, B. J. Chem. Eng. 2007, 15(3), 433-438.
CrossRef - Svetozar, M.; Israel, N. R.; Zvonko, O.; Stanko, P. Croat. Chem. Acta. 2004, CCACAA 77(1–2), 141-151.
- Singer, Ph. C.; Stumm, H. Am. Wat. Wor. Ass. 1970, 62(3), 198-202.
CrossRef - Mameri, Y. M. Sc. 2010, 163-174.
- Lee, S.; OH, J.; Shin B. Shigen-to-Sozai. 1999, 115, 815-819.
CrossRef - WANG, Z.; Chunchun, X.; CAO, X.; Ben, X. Chin. J. Chem. Eng. 2007, 15(3), 433—438.
CrossRef - Music, S.; Dragcevic, D.; Czakonagy, L.; Popovic, S. Croat. Chem. acta, 1997, 70(2), 689-702.
- Legrand, L.; Abdelmoula, M.; Géhin, A.; Chausse, A; Genin, J. M. R. Electrochim. Acta. 2001, 46(12), 1815-1822.
CrossRef - Jagannath, B.; Kyounglim, K.; Walter, D.C. S.; Susan, C. B.; Stephan, M. K.; Janet, G. H.; Stephan, J. H. Swiss Geo. Sci. Meeting. 2016, 14th, 133-140.
- Michaël, D.; Catherine, B.; Florence, M. G.; Sébastien, S; Joachim, S.; Pierpaolo, Z., Bull. Soc. géol. France. 2000, 3, 265-270.

This work is licensed under a Creative Commons Attribution 4.0 International License.









