Synthesis and Spectral Characterization of Verdantiol Schiff Base with GC-MS for Synthetic Ingredients in Fragrances Industry
Candra Irawan1, Dian Islamiyati1, Eva Yuliana2*, Singgih Wibowo1, Rika Perdana Putri3 And Imalia Dwi Putri1
1Departement of Chemical Analysis, Politeknik AKA Bogor, Bogor 16154, Indonesia
2Departement of Food Industry Quality Assurance, Politeknik AKA Bogor, Bogor 16154, Indonesia
3PT. Nilam Widuri Bogor, Indonesia
Corresponding Author Email: evayuliana@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350616
Article Received on : 06/11/2019
Article Accepted on : 06/12/2019
Article Published : 17 Dec 2019
In this study, we have synthesized verdantiol schiff base using a simple condensation method. The color of product has been compared with the verdantiol standard. There was a little color difference between the product and the standard. In order to confirm the product, gas chromatogram-mass spectrometer (GC-MS) has been used. The existence of verdantiol schiff base molecule ion (M+) was confirmed at m/z = 337.2 because this value was equal to the value of its molecular weight of 337.47 g/mol. This finding will be important for fragrance industry due to a simple method can be used to produce an important verdantiol schiff base.
KEYWORDS:Verdantiol; Schiff Base; GC-MS; Fragrance
Download this article as:| Copy the following to cite this article: Irawan C, Islamiyati D, Yuliana E, Wibowo S, Putri R. P, Putri I. D. Synthesis and Spectral Characterization of Verdantiol Schiff Base with GC-MS for Synthetic Ingredients in Fragrances Industry. Orient J Chem 2019; 35(6). |
| Copy the following to cite this URL: Irawan C, Islamiyati D, Yuliana E, Wibowo S, Putri R. P, Putri I. D. Synthesis and Spectral Characterization of Verdantiol Schiff Base with GC-MS for Synthetic Ingredients in Fragrances Industry. Orient J Chem 2019; 35(6). Available from: https://bit.ly/2rKcC6v |
Introduction
Personal care products such as soap, toothpaste, cosmetics, shamphoo and perfumes contain fragrances.1 Fragrance is a mixture of several chemical compounds which specifically produce aroma compounds.2,3,4,5 Fragrance material usually contains synthetic aroma compounds or extracted natural compound which is dissolved in alcohol.6 Fragrance quality can be seen from volatility, odor, stability, and color. 2,7,8
In order to produce high-quality fragrance, a synthetic compound such as Schiff base is added. Schiff base is a compound obtained from the reaction between aldehydes and primary amines. 9,10 In this reaction, water is obtained as a byproduct. This condensation reaction is reversible using acid or base catalysts with heating. Vacuum condition is applied to minimize the damage of schiff base products, water is separated from the products and high yield is obtained. 11,12 The amines compounds which are usually used in the fragrance industry are methyl anthranilate and ethyl anthranilate. While the commonly used aldehyde compounds are alkyl or aromatic aldehydes such as decanal, benzaldehyde, terpene aldehyde (hydroxycitronellal). 9,13
Schiff base obtained from methyl anthranilate is highly needed in fragrance industry. Schiff base is able to increase the chemical and temperature stability from fragrances.14 Several schiff bases have been using in fragrance industry such as Jasmea, Cyclantine, Indolen, Vertosine, Agrea, Decimea, Nonimea, , Ocmea, Isonomilat 258 E, Amandolen, Canthoxalia, Acaciol, Aurantiol, Lyrame and verdantiol14,15,16. Verdantiol is produced by condensation reaction of lilyall and methyl anthranilate14.
Mass spectrometry tandem with gas chromatography (GC/MS) is normally used for direct analysis of components existing in fragrance. In recent years GC-MS studies have been increasingly applied for the analysis of fragrance in the industry. This technique has proved to be a valuable method for the analysis of polar components and (semi) volatile, and only a few grams of sample are required.17,18,19
Experimentals
Materials and Methods
Methyl anthranylate, lilyall (aq) and ethanol 99% (v/v) were used as the main materials. The experiment consists of three steps (production, confirmation, and color observation) of verdantiol schiff base.
Production
Production of verdantiol schiff base was carried out by a simple condensation of method at a temperature of 110°C for 30 minutes using hotplate stirrer. The main composition of verdantiol schiff base is 9.070 g methyl anthranilate (aq) and lilyall (aq) (12.259 g) was added for obtaining verdantiol schiff base.
Confirmation
Schiff base products (0.05 g) were diluted with ethanol 99% (v/v) until the weight was 1 g. Then homogenized to produce 5% (w/w) solution. This schiff base solution was analyzed by gas chromatography–mass spectrometry (GC-MS). GC-MS was perfomed by using Agilent 5975C series MSD, capillary column (5% Phenyl Methyl Siloxane 325 °C, length=30 m, internal diameter = 320 μm, film thickness = 0.25 μm), carrier gas was helium, carrier gas pressure was 7.0531 psi, injector temperature was 100 °C, injection volume was 0,2 L, split injection technique with split ratio 80:1, the column temperature was 100°C for 5 min, the increment rate was 15°C/min, final temperature was 250 °C for 5 min. Total running time was 25 min.
Color Observation
The color observation was carried out by at least three panelists at the booth. The product solution is poured into a tube that is 1.3 cm in diameter and 10 cm high to the specified limit (about 5 cm). The product was also documented using a camera. Images are observed in places that have enough light, and there are no reflections of light.
Result and Discussion
Verdantiol Schiff Base Preparation
The obtained verdantiol schiff base can be seen in Figure 1. Figure 1 shows that the color of verdantiol schiff base is yellow, the standard color is more concentrated. Verdantiol schiff base is obtained from lilyall which has carbonyl group reacting with amine group in methyl anthranilate passing crossed aldol condensation reaction. If an aldehyde/ketone without α -hydrogen is mixed with aldehyde has 2 isomers those are R* and S*. The reaction occurs in the production of verdantiol schiff base is shown below (Figure 2).
 |
Figure 1: The obtained verdantiol (a) and verdantiol schiff base standard (b) Click here to View Figure |
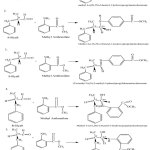 |
Figure 2: Schematic diagram of verdantiol schiff base formation. Click here to View Figure |
Analysis of Schiff Base Verdantiol using GC MS
Analysis of verdantiol schiff base product using GC MS obtained chromatogram and mass spectrum as seen in figure 3 and 4. Mass spectrum displays the number of molecule fragment which is produced from the fraction of chemical component having a different molecular weight.20 Analysis of mass spectrum is based on matching percentage value, base peak and the fraction of mass spectrum compared to the spectrum from Wiley.L. literature.
 |
Figure 3: (a) Verdantiol molecule chromatogram (b) Wiley.L literature for verdantiol molecule. Click here to View Figure |
Figure 3 shows verdantiol with molecular formula C22H27NO2 (molecular weight = 337.47 g/mol) with more than 90% similarity. The number of peaks is the reaction results between lilyall and methyl anthranilate which produce five different shapes of molecules. Theoretically, there are six isomers produced from the reaction between lilyall (R*,S*) with methyl anthranilate.
Chromatogram result from verdantiol molecule formation (Figure 2a) only shows 5 isomers. This might be caused by the structure of methyl 2-((1S,2R)-2-benzyl-1-hydroxypropylamino)benzoate which was not formed due to steric hindrance of a bulky group in a transition state. Bulky structure in the transition state causes activation energy getting very high.21
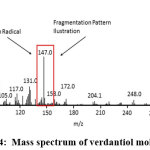 |
Figure 4: Mass spectrum of verdantiol molecule Click here to View Figure |
Mass spectrum in figure 5 shows peaks having different m/z with main peak at m/z of 337.2 and 147.0. McLafferty stated that the other peaks with the smallest abundance were smaller ion fraction radical and ion or radical small molecule.22 Molecule mass spectrum in figure 3 shows that the existence of verdantiol schiff base molecule ion (M+) at m/z = 337.2 because its value was equal to the value of the molecular weight of verdantiol schiff base which was 337.47 g/mol. The peak at m/z = 147 was the fragmentation from verdantiol. The following illustration shows the formation of molecule ion, basic peak and cation radical:
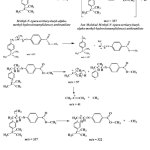 |
Figure 5: Fragmentation of verdantiol Click here to View Figure |
Conclusion
Verdantiol schiff base has been successfully synthesized by using a simple condensation method. The color of the product was yellow and almost similar to the standard color. However, the standard color was more concentrated compared to the product. From GC-MS analysis, the peak at m/z = 147 was the fragmentation from verdantiol.
Acknowledgements
This study was supported by Research Program of Politeknik AKA Bogor.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- Villa, C.; Gambaro, R.; Mariani, E.; Dorato,Journal of Pharmaceutical and Biomedical Analysis. 2007,44,755-762.
- Herman, S.Allured Publishing Corp. 2002. England.
- Ansari, R.M.; Bhat, B.J. Chem. Sci. 2017,129,1483–1490.
- Da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Cleide, V.B.; Martins, C.V.B.; de Fatima, A.Adv.Research. 2011,2,1–8.
- Nagesh, G.Y.; Mruthyunjayaswamy, B.H.J. Molecular Structure. 2015, 1085, 198-206.
- Rowe,Blackwell Publishing Ltd. 2005. USA.
- Quellet, C.; Schudel, M.; Ringgenberg,Chimia. 2001, 55, 421-428.
- Gygax, H.; Koch,Chimia. 2001, 55,401-405.
- Calkin, R.; Jellinek, J.S. John wiley & Sons, Inc. 1994. Canada.
- Dhokale, N.T.; Karale, B.; Nagawade, A.V. Orient. J. Chem. 2017, 33(1),165-172.
- Sarkic, A.; Stappen, I. Cosmetics. 2018, 5 (1),
- Kumar, Departement Pharmacy University of Punjab Technical Jalandhar. 2011. India.
- Cortez-Pereira, C.S.; Baby, A.R.; Velasco, M.V.R.Cosmetic Dermatology. 2010,9, 230–241.
- Sell, C.RSC Publishing. 2006. Dorchester.
- Irawan, C.; Indryati, S.; Lestari, E.S.; Hidaningrum, A.;Orient. J. Chem. 2018,34(1), 394-400.
- Irawan, C.; Islamiyati, D.; Putri, R.P.; Madiabu, M.J.; Supriyono. J. Chem. 2018, 34,3118-3122.
- Asten, A.TrAC-Trend Anal Chem. 2002, 21(9–10),698–708.
- Begnaud, F.; Chaintreau,Phil. Trans. R. Soc. A, 2016,374,20150365.
- Debonneville, C.; Chaintreau,J. Chromatogr. A, 2004, 1027, 109-115.
- Harianingsih, R.; Wulandari, C.; Harliyanto, C.N.; Techno, 2017.18 (1),23–27.
- Fessenden, R.J.; Fessenden, J.W. Grant Press. 1982. Boston, USA.
- McLafferty, F.University science books. 1980.California, USA.

This work is licensed under a Creative Commons Attribution 4.0 International License.









