Studies on Some of the Improvised Energetic Materials (IEMs): Detonation, Blast Impulse and TNT Equivalence Parameters
Kannan Gajendran Balachandar and Arumugam Thangamani*
Department of Chemistry, Karpagam Academy of Higher Education, Coimbatore-641 021, Tamil Nadu, India.
Corresponding Author Email: thangabell2003@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350626
Article Received on : 18-Oct-2019
Article Accepted on : 20-Nov-2019
Article Published : 25 Nov 2019
This work reports the computational analysis of the physicochemical, detonation, blast peak over pressure, blast impulse and TNT equivalence parameters of some of the Improvised Energetic Materials (IEMs) such as ammonium nitrate, urea nitrate, C4, hexamethylene triperoxide diamine (HMTD) and triacetone triperoxide (TATP), which are used in bombing incidents all over the world in the form of Vehicle-Borne Improvised Explosive Devices (VBIEDs) or Person-Borne Improvised Explosive Devices (PBIEDs). The blast impulse, peak over pressure, TNT equivalence and detonation parameters reported in this manuscript will be useful to assess the threat quotient caused by these IEMs, of great help for the energetic materials researchers, technologists and scientists to undertake further research work in the field and for the security agencies to understand the severity of the damage during explosion This paper also accounts for the available detection technologies to fabricate an explosive detection device for its effective identification and detection.
KEYWORDS:Ammonium Nitrate; Urea Nitrate; C4; Hexamethylene Triperoxide Diamine (HMTD); Triacetone Triperoxide (TATP)
Download this article as:| Copy the following to cite this article: Balachandar K. G, Thangamani A. A. Studies on Some of the Improvised Energetic Materials (IEMs): Detonation, Blast Impulse and TNT Equivalence Parameters. Orient J Chem 2019; 35(6). |
| Copy the following to cite this URL: Balachandar K. G, Thangamani A. A. Studies on Some of the Improvised Energetic Materials (IEMs): Detonation, Blast Impulse and TNT Equivalence Parameters. Orient J Chem 2019; 35(6). Available from: https://bit.ly/35B34bV |
Introduction
Peace in the world is disrupted in the name of religion, hatred, animosity, struggle for power, poverty, prosperity, economic aggression, political gains etc. The biggest threat to peace and co-existence is terrorism. Terrorism is an act by which a single person or an organization creates terror in the minds of common people to achieve his/its motive. Terrorism is wide spread and all nations are taking sincere efforts to curb the menace to save mankind from its clutches. The simple means of inflicting terror is by carrying out bomb blasts causing death and destruction. As per the FBI bomb data center, more than 71% of all terrorist activities indicate the use of explosives and incendiary agents in the form of bombs or Improvised-Explosive Devices (IEDs).
The global threat envisages a quick transformation from conventional improvised explosive devices to improvised chemical devices. Terrorists, extremists and antisocial elements often prefer IEDs because the ingredients, instructions and components to design IEDs are readily available. There is an urgent need to keep a close watch on the developments in the area of improvised energetic materials (IEMs), including the type of material reported in various literature and progress made by the world research community in the detection of improvised energetic materials. It is also important to take stock of the preventive measures and preparedness of the world community to face the threat of IEMs. It is also equally important to know how safely one can dispose of the detected IED filled with IEMs.
This paper discusses the traits of a few explosive ingredients extensively used by terrorists to fabricate bombs.
In the present scenario, a highly motivated person with a little knowledge of chemistry can fabricate a variety of bombs using some cumbersome mechanisms. The greatest advantage the terrorists have is that they can be prepared at home with simple available apparatuses and unrestricted commonly used precursor materials. According to United Nations International Ammunition Technical Guidelines (IATG)1, an IEM charge can be made from home-made explosives (Example from fertilisers containing nitrates), military explosives (such as ammunition diverted from storage or transport, or materials recovered from Explosive Remnants of War or Unexploded Ordnance, UXO) or civilian explosives (such as dynamite, trinitro toluene (TNT) or black powder).
Ammonium nitrate and urea nitrate are commonly available ingredients that can be used to make bombs easily. It is reported that ammonium nitrate and gelatin were used in several bomb blasts in Mumbai, India. In 13th July, 2011 Mumbai bombings, the National Investigation Agency found the use of IEDs based on Ammonium Nitrate Fuel Oil (ANFO) with some Molotov cocktails. C4 is a plastic explosive which is very challenging to detect. This explosive was used in U.S.S Cole incident in 2000 and Bali night club bombings in 2002. Hexamethylene triperoxide diamine (HMTD) and triacetone triperoxide (TATP) are frequently used peroxide-based explosives. TATP is used frequently because it is easy to make using household supplies. It is often prepared in makeshift laboratories found inside apartments, homes and other residential buildings. Oklahoma City bombings in 1995, blasts in Paris in 2000, bombings and suicide attacks in Brussels 2016, Manchester arena bombings in 2017, explosion in Barcelona in 2017 and Easter blasts in Srilanka in 2019 are instances where TATP was used. Apart from that, “the shoe bomber and underwear bomber” were evidences of the merits of TATP that can pass security checks without detection2.
In spite of various advanced identification technologies, on February 2, 2016, a terrorist was able to board a plane with a sophisticated laptop bomb creating havoc. In October 2010, an innovative bomb maker constructed a very difficult-to-identify IED, involving printer cartridges using PETN. Security agencies in the airport failed to identify or detect it either by x-ray scanner or by sniffer dogs. It was recovered only through human intelligence. The advanced imaging technology (AIT) machines would detect surgically implanted devices because of the signs of incisions but failed to identify the IEDs implanted in rectum3. The challenges in identifying and diffusing these bombs cannot be overcome until the physicochemical properties of these energetic materials are fully known to the researchers and the security forces working on it. The physical and chemical characteristics, detonation properties, blast peak over pressure and blast impulse have a role in the design of an explosive detection device. The detonation behavior of TATP, HMTD and ANFO was investigated, involving thermo-chemical calculations using kinetic CHEETAH code and reactive flow models using AUTODYN code4. Studies about the detonation properties, relative performance, blast effects and shock waves were done for the explosive mixtures of TATP and ammonium nitrate with added water and for the mixture of TATP and urea nitrate by means of Cheetah code5.
On occurrence of a blast, the primary task of the investigating agency is to ascertain the type and amount of explosive used. This can be achieved by estimating the TNT equivalence (TNTeq). TNTeq represents the weight of TNT, which yields the same amount of energy as a mass unit of the considered explosive. It is used to express the power of the nuclear weapons in kilotons and megatons. The blast effects of a large number of secondary explosives are characterized by comparing them with the effects of TNT. TNTeq is dependent on scaled distance. The results of TNTeq from blast peak over pressure are higher than those from the blast impulse. TNTeq is very much essential to estimate the effective explosive weight (EEW), which is a basic parameter to determine the safety distances. There are numerous methods to determine TNT equivalence, but they do not yield the same values. TNT equivalence for an explosive varies with distance. According to DOD standard, TNTeq is determined by the comparison of values of blast over pressure and impulse of various explosives to TNT6,7.
An overview of IEMs
Typical Compositions of Improvised Energetic Materials (IEMs)
IEMs characteristics1:
They contain organic or inorganic salts with energetic groups such as, perchlorate, chlorate, nitrate etc. in combinations, along with fuel functional groups such as -CH2. They can also be derived from the existing organic substances with nitramine and nitro- nitroxy-compounds or their combinations with fuel elements in them.
They can be based on peroxides that may be easily produced from the easily available raw materials or chemicals of dual use (Example: TATP). Combustible dusts or fuels can be used in the surroundings of the containers in order to produce deflagration.
They can also be based on “liquid explosives” composed of oxidizers (such as nitric acid and nitro compounds or aromatic liquids) along with fuel-value raw materials.
Types of Improvised Explosive Materials
The IEMs are classified into five categories based on the physicochemical properties of the high explosives8.
IEMs from precursor compounds
The IEMs that are prepared from easily available components fall in this category. They are cheap and possess minimum detonating mass. These IEMs also possess an average density in the range of 0.85-1.3 g/cm3. These types of IEMs are extremely unstable, highly unpredictable and may detonate spontaneously during handling, storage and transportation. They possess high vapour pressure and high volatility and possess shorter shelf life. They may be stored under suitable conditions along with other suitable raw materials in order to improve their shelf life. These materials are also highly sensitive to moisture (Example: TATP, HMTD, etc.).
IEMs from Military explosives
The antisocial elements who have access to military explosives prepare improvised energetic materials with different combinations, which are reported to possess density of the order of 0.8-1.1 g/cm3 (Example: Chechen mix: such as ammoniac saltpetre, sugar, aluminium powder, and RDX).
IEMs from civilian application explosives and pyrotechnics
These types of IEMs are very easy to produce on the ground and the raw materials are easily available and inexpensive. The power output of these IEMs is believed to be half of the well-known explosive trinitrotoluene. The average density is of the order of 0.8-1.1 g/cm3. The IEDs of this category have detonating mass of more than 1000 grams. They also require an appropriate packing in order to increase their shelf life (Example: ammonals).
IEMs from industrially manufactured commercial explosives
These IEMs are based on the materials used in commercial or civil applications. They may be derived from military energetic materials along with fuel components such as ammonia. They are energetic in nature with higher detonation performance and may possess densities of the order of 0.85-1.25 g/cm3. During the manufacture of IEMs, generally, marking agents are added by the industries for ease of detection (Example: Grammonite 50/50, Ammonite).
IEMs from energetic materials of military ordnance
The last category of IEMs is based on the military energetic materials which are industrially produced. These categories of IEMs are generally stable in nature and possess regulated sensitivity to external stimuli. They may possess density of > 1.5 g/cm3.
Constituents of the improvised energetic materials
IEMs are produced either by blending or by cooking. Blending is the physical mixture of precursor materials in which there is an oxidizer mixed with a fuel (an example of blending is ANFO). Here, ammonium nitrate is combined with a fuel oil to form the explosive mixture. Cooking is the process where multiple precursor chemicals are mixed together and allowed to react chemically to form an explosive material; and a few examples are TATP, urea nitrate and ethylene glycol dinitrate (EGDN). They are also called synthesized explosives9,10.
The IEDs’ design and types
An improvised explosive device (IED) is a type of unconventional bomb that can cause loss of life, property and injury both in civilian and military environment. An IED contains four major components such as Main charge, Mechanism, Power Source and a Detonator. The explosive or energetic materials, either factory made or improvised, constitute the main charge. There are different kinds of mechanisms, either standard or improvised. The maker of the Improvised-explosive device can design his own mechanism depending on the availability of resources. There can also be more than one mechanism in a single IED. Broadly it is classified into four types such as Anti-handling, Delay, Ambient and Command mechanisms. Command wire IEDs and Pressure-Plate IEDs are frequently used. The power source may be electric or non-electric. Normally, packet C-type batteries (output voltage 1.5 V) connected by a two-wire cable is used in Pressure-Plate IEDs (PPIEDs), and alternating current sources such as generators or chargers providing voltage of 110V- 220V are used in Command Wire IEDs. They are connected to the power source by cable line of 100-250 m length in a concealed manner. There are two types of IEDs. The first one is the open type, where the explosives and the mechanism can be seen through the naked eye and the second one is the closed type in which the explosives and mechanism are concealed and cannot be visualized. The IEDs targeting individuals are called Person-borne IEDs (PBIEDs), those targeting vehicles are called Vehicle-borne IEDs (VBIEDs) and those targeting aircraft are called Air-borne IEDs (ABIEDs)11.
The scourge of IEDs
IEDs are the most pernicious, harmful and widespread of explosive weapons used against humanity. Data based on ‘Action on Armed Violence (AOAV) recorded 74% of the reported casualties were civilians. They massively elevate the threat to civilians because 21% of the IED incidents occurred in crowded areas like markets, places of worship etc. The majority of deaths caused by IEDs occurred in Afghanistan, Iraq, Egypt, India, Nigeria, Pakistan, Philippines, Somalia, Syria, Thailand, Turkey and Yemen in incidents varying in the number from 100 to 3000 per year. The ISIS remains the most prolific user of IEDs. Car bombs, road side bombs, suicide bombings, Victim-activated IEDs (VAIEDs), drones, robot bombs are based on methods of delivery. Car bombs with a great payload capacity are proved most destructive with an average of 30 casualties, and roadside bombs with small payloads cause an average of 6 casualties. VAIEDs which are de facto antipersonnel mines, banned under international humanitarian law, account for 16% of IED incidents mostly against the security forces. Robot-bomb prototype comprising a Remote with C4 explosive killed a sniper. Using drones with explosives to target political leaders is a new method of assassination. In 2016, the United Nations Secretary-General released a report “Countering the threat posed by Improvised explosive devices”, called for an effective response, and encouraged greater research in the explosives domain to tackle the reverberating effects of IEDs12-15.
Experimental Section
Preparation of IEMs
Due to its sensitive nature, authors have not given any information relating to the actual method of preparation/synthesis procedure/manufacturing methods of IEMs. However, the information available in literature regarding the widely used IEMs is discussed.
Ammonium nitrate
Ammonium nitrate is a commonly available chemical compound which can be used to make effective IEDs. It is used in fertilizers and available in agricultural supply stores. The reason for the extensive use of ammonium nitrate is that it contains three parts of oxygen16. Ammonium nitrate is highly hygroscopic. In a few cases of IEDs in India, the bomb detection and disposal squad (BDDS) experts suggested that dampness in weather have nullified the intensity of blasts. IED containers containing ammonium nitrate explosives are covered with plastic film for safeguard against moisture as they can absorb ambient moisture or even bind water11. Ammonium nitrate is used in different blends such as ammonium nitrate fuel oil (ANFO), ammonium nitrate aluminium powder (ANAL), ammonium nitrate with sulphur powder (ANS) and ammonium nitrate with icing sugar (ANIS) in preparing improvised explosives.
Ammonium nitrate fuel oil (ANFO)
ANFO stands for ammonium nitrate fuel oil. Timothy McVeigh used ammonium nitrate mixed with fuel oil to destroy a murrah federal building in Oklahoma City. ANFO is widely used by American agriculturists for clearing the stumps of trees to convert forests into cultivable land. Fuel oil is used as both fuel and heating source in farm tractors. Protection is given by coating the prills of ammonium nitrate to avoid absorbency of fuel oil, but still, bomb makers find novel methods to overcome them. Ammonium nitrate when mixed with model engine fuel such as nitro-methane, a chemical with explosive properties greater than TNT, was used to destroy government quarters in Oslo, Norway in 2011.
Ammonium nitrate powder and aluminum powder (ANAL)
Ammonium nitrate obtained from fertilizer stores and aluminum powder from paint stores were mixed to form an explosive mixture. It is very powerful and possesses 75% of the power of TNT. It is used as main charge and requires a booster to carry out the detonation. Aluminum powder is used to enhance the output of heat. ANAL mixed with TNT is called Ammonals. It has a few limitations: it is highly sensitive to friction and impact and its electric-charge sensitivity is very high. It requires only a blasting cap for detonation. Such devices are especially used as water-based slurry explosives. The velocity of detonation ranges from 3125 to 4575 m/s, depending on the grade and configuration17. This improvised mix of explosive is used in plenty by Irish, Spanish, Chechen, Saudi Arabian and Kashmir terrorists. Tannerite is the brand name of a substance made of ANAL.
Urea Nitrate
Urea nitrate is used in fertilizers for agricultural farms, plastic industry and as chemical deicers. It is highly corrosive and due to its acidic nature, it will react with metals. Hence, the improvised explosive made of urea nitrate is not safe. It is also known as acidogen nitrate. Urea nitrate is frequently used as a main charge secondary explosive and needs a booster to carry out detonation due to its insensitivity. It is not sensitive to shock, heat or friction. While on improvisation, accidents may occur, because both the ingredients are corrosive. IEDs are not made using metal containers because of urea nitrate’s acidity that interacts with metals16. In 1993 World Trade Center attack, more than 680 kg of urea nitrate was used. Foiled attempts were reported in Australia, US and the Middle East about the use of urea nitrate in chemical explosion devices.
Hexamethylenetriene triperoxide diamine (HMTD)
Hexamethylene triperoxide diamine is a powerful peroxide and can be synthesized using precursor chemicals available from common stores. It has the power of 60-116% of TNT and contains peroxide, citric acid and hexamine as ingredients (Scheme 1). Hexamine or urotropine is found in fuel tablets. Their explosive property is intact up to 7 to 10 days of preparation and shows an appreciable decomposition after that. It can be used as main charge and as improvised blasting cap because of its excellent initiating property. It is highly sensitive to heat, shock and friction, but less sensitive than TATP. It is in the form of white powder or crystals, insoluble in organic solvents and water and causes metallic corrosion. The peculiarity is that its stability starts below 70 F and hence must be stored in a cooler, refrigerator or an insulated container18. In the 1999 millennium bombing plot, Ahmad Ressam used HMTD in an IED to attack LAX airport. The recent Chelsea bombings used HMTD as main charge.
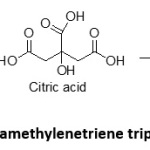 |
Scheme 1: Synthesis of hexamethylenetriene triperoxide diamine (HMTD) Click here to View Scheme |
Triacetone triperoxide (TATP)
TATP stands for triacetone triperoxide which is a common explosive manufactured by combining acetone, which is used in nail-polish remover, hydrogen peroxide, which is used in hair dye and sulphuric acid used as drain cleaner and battery acid, in the presence of hydrochloric acid used as a catalyst and kept at around 0°C (Scheme 2). It is also called the ‘Mother of Slave” or “Mother of Satan” by terrorists, because it is very dangerous to handle. Two high-profile terrorist attacks using TATP at Paris in November 2015, and at Brussels in March 2016, showed the world how it was going to be a global threat in future. It is a cyclic trimer of acetone peroxide in which peroxide bonds forming a highly strained nine-member ring give the molecule its high level of instability, because of which it is extremely sensitive to heat, friction, shock or impact. The explosion is not a thermodynamically favoured one, but it releases huge energy because of an entropic burst due to the strained system in which the peroxide bonds are broken to release ozone and three acetone molecules. It looks like a white granular powder and has an acrid smell. It has 88% of the power of TNT and can be used as a booster or as a main charge. TATP shock wave overpressure is 70% of that for TNT and positive phase impulse is 55% of TNT equivalent. TATP has one-third of the brisance of TNT. The peculiarity of TATP is that at higher concentrations it can emit strong vapours and can detonate at higher temperatures. Being a non–nitrogenous compound, it is nearly impossible to detect using nitrogen-based detection devices17,19. Accordingly, in December 2001, Richard Reid, a shoe bomber, used TATP and tried to blast an airplane without a detonator. It has a short shelf-life of nearly 10 days. Currently this is the common explosive used by Hamas terrorists in Israel. Apart from the above said two attacks, TATP was also used in 2003 Casablanca bombings in Morocco and London subway bombings in 2005.
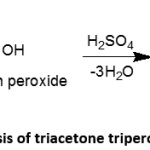 |
Scheme 2: Synthesis of triacetone triperoxide (TATP) Click here to View Scheme |
The stability and sensitivity of an energetic material is generally determined by its weakest bond20 in its trigger linkage (Table 1). Commonly, the C-NO2 functional group is present in the nitrated explosives, whereas peroxide functional group contains -O-O- bond. The shelf life and the sensitivity of IEMs are determined by the presence of the type of bond in it. The bond energies of the weakest bond are given below:
Table 1: Bond energy and activation energy of various types of functional groups
|
No. |
Type of Compound | Trigger Bond Linkage | Bond Energy (kcal/mol) | Activation Energy (kcal/mol) |
|
1 |
Peroxide | -C-O-O-C- | 34 |
35 |
|
2 |
Nitrate esters | -CO-NO2 | 53 | 40 |
| 3 | Nitramines | -N-NO2 | 39 |
47 |
| 4 | Nitroarenes | -C-NO2 | 73 |
70 |
It is very clear from the above table that peroxides are the most sensitive and dangerous of all substances among all the category compounds given above.
TATP has power close to TNT, and as one of the most sensitive explosives known, it is used both as a main charge and primary explosive. Peroxide-based explosives, such as triacetone triperoxide (TATP) and diacetone diperodixe (DADP), are rarely utilised either in military or in civilian applications because of their high volatility, high sensitivity to mechanical impact, open flame and low chemical stability. Unfortunately, the easy availability of materials through which these peroxide-based compounds can be synthesized straightaway, has made them popular among terrorists worldwide. Terrorists are successful in preparing rightful combinations to carry out a few blasts based on these peroxide-based explosives. Unlike the most conventional explosive devices, the improvised explosive devices made of peroxide-based energetic materials contain neither metallic elements nor nitro groups, making it difficult to detect by standard methods. This drove the security personnel and the scientific community towards the search for an analytical methodology equipped to detect these peroxide-based materials. An extensive understanding of the initial chemical developments that leads to the initiation of these energetic materials is critical to carrying out the desired remote detection.
C4 or Plastique
C4 stands for the 4th generation of Composition C explosives- an American name. It is also termed as Harrisite. It contains 90% of RDX and 10% of plasticizer. Normally 5.3% dioctyl sebacate or diictyl adipate is used as plasticizer, 2.1% polyisobutylene as binder along with 1.6% of mineral oil. It is a light brown putty like substance and smells like motor oil. Various countries fabricate their own C4. 2,3-dinitro-2,3-dimethyl butane used as taggant and low molecular mass hydroxy terminated polybutadiene (HTPB) is used as a binder for prolonged storage. It is insensitive to shock, non-toxic and will burn and ignite. It is used in metal cutting and demolition. It is widely used for underwater explosions. Improvised C4 is prepared using three ingredients such as ammonium nitrate, nitromethane and denatured ethyl alcohol. Ammonium nitrate is obtained as fertilizer from agricultural stores. Nitromethane is a washing solvent which is used to dissolve plastics, clean up fat, wax and be a fuel additive. It can be obtained from petroleum dealers and hobby shops. Denatured ethyl alcohol can be purchased from paint-supply stores. Producing C4 substitute is not dangerous or difficult, but some simple procedure has to be followed. Too much of nitromethane will kill the mixture and too little will not be sufficient to sensitize ammonium nitrate. Hence, one-part nitromethane to three parts of ammonium nitrate by volume, or two parts of nitromethane to five parts of ammonium nitrate by weight, is the optimum quantity to synthesize C4. The velocity of detonation of an improvised C4 is 8.052 km/s or slightly less than TNT. This improvised C4 is remarkably similar to genuine C4 in stability, making it formidable21. This is a preferred explosive of terrorists. This is recommended in Al-Qaeda’s traditional curriculum of explosive training and often used in Iraq.
The physicochemical properties of the listed compounds are enumerated in the Table 2 below. From the Table 2 it is inferred that the density is greater for ammonium nitrate and it also has the positive oxygen balance. The detonation velocity is high for urea nitrate. Though the peroxide-based explosives possess low detonation velocity, it is preferred due to its peculiar features. C4 possesses the highest detonation pressure and gurney velocity.
Table 2: Physicochemical properties of the selected IEMs
|
Properties |
Ammonium Nitrate | Urea Nitrate | C4 | HMTD | TATP |
| Molecular Formula | H4N2O3 | CH5N3O4 | C3H6N6O6 | C6H18N2O6 |
C9H18O6 |
|
Density (g/cm3) |
1.72 | 1.59 | 1.65 | 1.57 | 1.272 |
| Molecular Weight | 80 | 123.07 | 222 | 214 |
222 |
|
Oxygen Balance (%) |
20 | -6.5 | -46.4 | -92.2 | -151.2 |
| Detonation Velocity (m/s) | 6768 | 7442 | 7763 | 6545 |
5723 |
|
Detonation Pressure (kbar) |
151 | 195 | 243 | 167 | 101 |
| Gurney Velocity (m/s) | 1549 | 2081 | 2645 | 2353 |
2072 |
|
Heat of Formation (kJ/mol) |
-365 | -546 | -13.93 | -359.99 | -395.47 |
| Detonation Temperature (K) | 1619.95 | 2388.9 | 3711.71 | 3137.12 |
2709.55 |
|
TNT Equivalence |
0.74 | 1.05 | 1.34 | 0.74 |
0.80 |
In view of the above, and in continuation of our work in the area of energetic materials, we report the physicochemical properties, detonation properties, and blast peak over pressure and blast impulse of some of the most widely used IEMs such as urea nitrate, C4 or plastique, hexamethylene triperoxide diamine (HMTD) and triacetone triperoxide (TATP) (Figure 1).
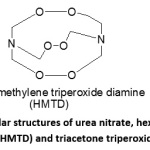 |
Figure 1: Simple molecular structures of urea nitrate, hexamethylene triperoxide diamine (HMTD) and triacetone triperoxide (TATP). Click here to View Figure |
Results and Discussion
TNT equivalence
TNT equivalence is an expression to compare the effects of the output of a given explosive material to that of TNT. It is defined as the measure of the energy released from the detonation of a nuclear weapon or from the explosion of a given quantity of explosive material in terms of amount of TNT, which would release the same amount of energy when exploded. TNT equivalence accounts for the amount of pure semi-spherical shaped charge of TNT, which, during detonation on the ground level at precise distance, yields the same blast over pressure or blast impulse as the mass unit of the considered explosive. It is used to predict the blast waves, craters, structural response and frame government guidelines for handling and storage of explosive substances and for the siting and design of explosive facilities22,23. From Table 2, it is inferred that C4 has the highest TNT equivalence among the frequently used IEMs. The simulated result using Cheetah code gives the TNTeq values as 0.92 for TATP and 0.82 for HMTD [4]. The calculated TNTeq values 0.80 and 0.74 for TATP and HMTD have small deviations from the Cheetah code. The TNT equivalence enumerated in Table 2 of the selected molecules can be used to evaluate the explosive strength.
Prediction of Peak over pressure and Blast impulse
The numerical simulation of the advanced IEMs was carried out using SPEED, a hydro code24. In this modelling study, explosive charge quantity of 2 kg was considered for the prediction of blast peak over pressure and blast impulse. The title compounds viz., ammonium nitrate, urea nitrate, C4, HMTD and TATP were considered for modelling studies. It is clear from Table 3 that the blast peak over pressure and blast impulse increase with a decrease in the distance. High velocity of detonation results in the release of a high amount of energy which is in direct proportion to the blast peak over pressure and blast impulse20. From Table 3, it is inferred that C4 has the highest detonation velocity and accordingly it has the highest peak over pressure and blast impulse. Pure or ideal explosives have both fuel and oxidizer bonded at molecular level and hence the blast wave impulse and peak over pressure are in proportion to the yielded detonation energy, whereas in non-ideal explosives both the components are mixed and the degradation of the explosive occurs beyond the zone of chemical reaction and hence the blast wave impulse and peak over pressure are not in proportion to the yielded detonation energy. For the compounds such as ammonium nitrate fuel oil (ANFO) and C4, which are non-ideal explosives, the blast wave impulse and peak over pressure are not in proportion to the yielded detonation energy. During explosion, the blast over pressure accounts for damage to the objects involving ideal explosives, and it accounts for throwing of objects involving non-ideal explosives.
Table 3: Blast peak over pressure and blast impulse data predicted for the selected IEMs
|
Compound |
Charge Quantity: 2 Kg | Blast POP (bar) | Blast Impulse (bar. ms) | |||||||||
|
Distance from Charge |
Distance from Charge |
|||||||||||
| Density (g/cm3) | HOF(kJ/mol) | VOD (m/s) | Pcj (kbar) | 2 | 3 | 4 | 5 | 2 | 3 | 4 |
5 |
|
|
TNT |
1.62 | -67 | 6700 | 190 | 3.51 | 1.37 | 0.72 | 0.45 | 0.95 | 0.67 | 0.5 | 0.38 |
| HMX | 1.91 | 75 | 8700 | 393 | 4.03 | 1.52 | 0.82 | 0.53 | 1.24 | 0.95 | 0.75 |
0.62 |
|
HMTD |
1.57 | -359.99 | 6545 | 167.775 | 3.61 | 1.39 | 0.76 | 0.49 | 1.17 | 0.88 | 0.7 | 0.57 |
| TATP | 1.27 | -395.47 | 5723 | 101.110 | 3.52 | 1.34 | 0.75 | 0.48 | 1.15 | 0.87 | 0.68 |
0.56 |
|
C4 |
1.65 | -13.933 | 7763 | 243.014 | 3.49 | 1.35 | 0.74 | 0.48 | 1.14 | 0.87 | 0.68 | 0.56 |
| Urea Nitrate | 1.69 | -546.47 | 7442 | 195.542 | 2.53 | 1.04 | 0.58 | 0.39 | 0.93 | 0.7 | 0.54 |
0.44 |
The peak over pressure and blast impulse computed using SPEED software is depicted in Table 3. The predicted data have also been depicted in the form of Figures 2-11 by depicting graphs peak over pressure Vs time and blast impulse Vs time. The calculated blast impulse and peak over pressure data indicate that as the distance from the charge increased, the decrease in the effect was noticed in terms of lethality of blast and peak over pressure impact in the surrounding area.
The availability of the blast and peak over pressure data obtained from high speed computational thermochemical codes enables the explosives engineers/technologists and scientists to optimize building layouts and blast walls surrounding the storage facility. This will further help in protecting the civilian and military infrastructure surrounding the storage sites in case of accidental or unintended initiation of the weapon system. The proposed buildings must have sufficient ductility and redundancy to withstand/prevent the progressive collapse of the blast wave in accordance with the safety distances proposed in the STEC pamphlet guidelines. An internal layout structure of the building must be such that it should allow the escape of hot gases generated after internal explosion, and prevent the channelling effect due to successive shock wave generation and reflections. The explosion of the designed molecules may result in the sudden and rapid release of a high amount of energy, whereas metallized explosives of designed explosive molecules and their composition may undergo violent explosion resulting in the formation of hot gases and may create multiple layers of compressed air and shock wave expansion of the hot gases from the epicentre thus creating a layer of compressed air and shock wave. The influence of the shock wave reflections on the pressure was also analysed. The quantity of the explosives detonated is directly related to the magnitude of the explosion. The energy equivalence of the newly designed molecules is compared with the conventional energetic material trinitro toluene (TNT).
The shape of the explosive’s charge plays a pivotal role in defining the shock front, which is very important in the analysis of a close-range explosion processes. The most commonly used explosives charges by most of the researchers are spherical charges. In the current study also, a spherical charge of two kilogram was used for the analysis of blast impulse and peak over pressure of the designed molecules.
In case of spherical charges, generally the shock waves generated after the explosion, expand radially in the air from the detonation point. It is also very much essential to have previous knowledge about the shock wave behaviour to evaluate blast loading on the surrounding civilian/military infrastructure. During the speed analysis of the shock waves the important properties considered are arrival time of the shock wave, the incidental pressure and the duration time of the positive phase.
The performance parameters of the IEMs were calculated using EXTEC programme. It is very clear from Table 3 that the performance of urea nitrate and C4 IEMs is far better than that of TNT in terms of velocity of detonation. The CJ pressure of urea nitrate is lower than that of the C4 due to the presence of cool gases such as nitrogen in the post-detonation products.
 |
Figure 2: The graph of peak over pressure Vs time for HMTD Click here to View Figure |
 |
Figure 3: The graph of blast impulse Vs time for HMTD Click here to View Figure |
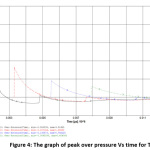 |
Figure 4: The graph of peak over pressure Vs time for TATP Click here to View Figure |
 |
Figure 5: The graph of blast impulse Vs time for TATP Click here to View Figure |
 |
Figure 6: The graph of peak over pressure Vs time for C4 Click here to View Figure |
 |
Figure 7: The graph of blast impulse Vs time for C4 Click here to View Figure |
 |
Figure 8: The graph of peak over pressure Vs time for urea nitrate Click here to View Figure |
 |
Figure 9: The graph of blast impulse Vs time for urea nitrate Click here to View Figure |
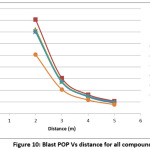 |
Figure 10: Blast POP Vs distance for all compounds Click here to View Figure |
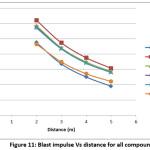 |
Figure 11: Blast impulse Vs distance for all compounds Click here to View Figure |
Sensitivity and Stability
Sensitivity parameter is a must before the actual fabrication of the explosive materials to nullify the occurrence of accidents or to diffuse them. It denotes the intensity of shock, friction, or heat required for an explosive to be ignited or detonated. The peroxide-based explosives are found to be highly sensitive and highly instable. The impact sensitivity of TATP is 0.3 Nm and HMTD is 0.6 Nm and the friction sensitivity is less than 0.1 N for both TATP and HMTD4.
Latest Detection Technologies
There are multi-layered screening processes to identify the presence of improvised or factory-made explosives. The most commonly used explosive detection systems are AIT (body scanners), door frame metal detectors (DFMD), and hand-held metal detectors (HHMD), deep search mine detectors (DSMD), multi-view X-ray machines in conjunction with explosive trace detection (ETD) systems and canine teams. The greatest fallacy in explosive detection is that the DFMD, HHMD and DSMD simply detect the anomalies or the objects that are not in the right place. X-ray does not account for specific detection or chemical identification, whereas it identifies the object inside the package with density variation or mass comparison. The ultimate 3D X-ray imaging system uses computed tomography (CT) approach to create a pseudo-3D imagery in advanced systems. Explosive trace detection systems are based on the method of separation and detection commonly known as ion mobility spectrometers (IMS). The wide range of molecular properties that govern the size and shape of chemical explosives is used in ETD technologies for detection. IMS have distinct advantages. They are highly sensitive systems and can detect nano-gram level of explosive traces through ionization process. They can operate in ambient conditions and be readily deployable for intended application at a desired place. They are excellent in detecting TNT, plasticized explosives like C4, PETN and Semtex when used in combination with X-ray technologies. ETD technologies are also capable of detecting the latest and much talked threat of TATP, when interfaced with triple quadrapole mass spectrometer25,26. TATP synthesis in large scale can also be identified by excessive bleach-like or fruity smells. The 2016 Brussels bombings report mentions that a person who makes TATP “smells like chemicals”. Canines are trained to identify explosives in compound forms and not as pure materials. They are trained to sniff the more volatile scents that emanate from the explosive mixture such as binders, solvents and plasticizers used in the formulation of explosives. Pure explosive substances do not emit vapour in enough concentration to be identified by canines. A team of minimum 4 dogs is required to segregate 6 different types of explosives for an effective canine squad in sanitising an area of threat. Canine detection is effectively presumptive rather than confirmatory tests.
Conclusion
In this paper, we dealt the physicochemical and explosives’ properties, the blast peak over pressure and blast impulse and the challenges and technologies in the detection of five frequently used improvised explosives. This will create a better understanding of IEDs made out of the said explosives and pave better research and advancement in the field of detection and disposal of these IEDs to thwart the threat they pose to the society. The data reported in this work further enriches the understanding of scientific data in specific performance parameters, peak over pressure and blast impulse data. The C4 and urea nitrate showed better performance than HMTD or TATP. The data also make value addition to the researchers and technologists working in the field of energetic materials, and provide a safety indication for maintaining safe distances during the diffusion or destruction of the IEDs filled with these improvised energetic materials.
Acknowledgement
Authors thank HEMRL, DRDO Lab, Pune, India for supporting EXTEC and SPEED software calculations.
References
- Sareva, J. Addressing Improvised Explosive Devices Options and Opportunities to Better Utilize UN Processes and Actors, UNIDIR and the Graduate Institute of International and Developmental Studies, New York, 2015.
- Horton, D. K. Morb. Mortal. Wkly. Rep. 2013, 62, 498-500.
- Liscouki, R.; McGann, W. CTC Sentinel 2016, 9, 1-6.
- Price, M. A.; Ghee, A. H. Cent. Eur. J. Energ. Mater. 2009, 6, 239-254.
- Zeman, S.; Trzcinski, W. A.; Matyas, R. J. Hazard. Mater. 2008, 154, 192-198.
- Pachman, J.; Matyas, R.; Kunzel, M. Shock Waves 2014, 24, 439-445.
- Jeremic, R.; Bajic, Z. Sci. Tech. Rev. 2006, 6, 58-62.
- UNMAS (United Nations Mine Action Service), Improvised Explosive Device Lexicon, New York, United Nations 2016.
- Greenfield, V. A. Reducing the Threat of Improvised Explosive Device Attacks by Restricting Access to Explosive Precursor Chemicals, The National Academies Press, Washington DC, 2018, p. 23-36.
- Bomb Making Materials Awareness Program (BMAP), Homeland Security, USA 2018.
- Motrycz, G. Szybkobiezne Pojazdy Gasienicowe 2017, 44, 95-108.
- Overton, I. Improvised Explosive Device Monitor, in: The Global Burden of Improvised Explosive Devices (Eds.: I. Overton, J. Dathan), Armed Violence, Ministry of Foreign Affairs, Norway 2017, p.1-14.
- Overton, I. Improvised Explosive Device Monitor, in: The Islamic State’s Suicide Industry (Eds.: C. Winter and J. Whittaker), 2017, p. 15-18.
- Overton, I. Improvised Explosive Device Monitor, in: Drones and the IED Threat (Eds.: R. Davies MBE QGM), 2017, p. 19-25.
- Overton, I. Improvised Explosive Device Monitor, in: The Evolution of Suicide Car Bombs Examined (Eds.: H. Kaaman), 2017, p. 26-31.
- Rostberg, J. I. Common Chemicals as Precursors of Improvised Explosive Devices: The Challenges of Defeating Domestic Terrorism, M. A. Thesis, Naval Postgraduate School, Monterey, CA, 2005.
- Technical Resource for Incident Prevention, Homeland Security, USA, 2018.
- Gu, W.; Jiang, J. J.; Jiang, J. C.; Bin, L. IOP Conf. Ser.: Earth and Environ. Sci. 2018, 189, 032046.
- Oxley, J.; Smith, J. Detection and Disposal of Improvised Explosives, in: Peroxide Explosives (Eds.: H. Schubert, A. Kuznetsov), The NATO Programme for Security Through Science, Springer Publications, 2006, p. 113-121.
- Cooper, P. W. Explosive Engineering, Wiley-VCH Verlag GmbH, Weinheim, Germany, 1996.
- Benson, R. Homemade C-4: A recipe for Survival, Paladin Press, USA, 1990.
- Cooper, P. W. Comments on TNT equivalence, 20th International Pyrotechnics Seminar, Colorado & Co, USA, 1994.
- Maienschein, J. L. TNT Equivalency of Different Explosives-Estimation for Calculating Load Limits in Heat Firing Tanks, Lawrence Livemore National Laboratory, 2002.
- SPEED (Shock Physics Explicit Eulerian/Lagrangian Dynamics), 3.2.1, Numerics Software, Developed by NUMERICS Software GmbH-Mozartring 3-85238 Petershausen, Germany, 2019. E-Mail: info@numerics-gmbh.de,Web site: www. numerics-gmbh.de.
- Beveridge, A. D. Forensic Sci. Rev. 1992, 4, 17-49.
- Tomlinson-Phillips, J.; Wooten, A.; Kozole, J.; Deline, J.; Beresford, P.; Stairs, J. Talanta 2014, 127, 152-162.
Symbols and Abbreviations
| IEMs | Improvised energetic materials |
| VBIEDs | Vehicle-borne improvised explosive devices |
| PBIEDs | Person-borne improvised explosive devices |
| HMTD | Hexamethylene triperoxide diamine |
| TATP | Triacetone triperoxide |
| FBI | Federal bureau of investigation |
| IEDs | Improvised-explosive devices |
| IATG | International ammunition technical guidelines |
| UXO | Unexploded ordnance |
| TNT | Trinitro toluene |
| ANFO | Ammonium nitrate fuel oil |
| PETN | Pentaerythritol tetranitrate |
| AIT | Advanced imaging technology |
| EEW | Effective explosive weight |
| DOD | Department of defense |
| RDX | Research department explosive |
| EGDN | Ethylene glycol dinitrate |
| PPIEDs | Pressure-plate improvised explosive devices |
| ABIEDs | Air-borne improvised explosive devices |
| AOAV | Action on armed violence |
| VAIEDs | Victim-activated improvised explosive devices |
| BDDS | Bomb detection and disposal squad |
| ANAL | Ammonium nitrate aluminium powder |
| ANS | Ammonium nitrate with sulphur powder |
| ANIS | Ammonium nitrate with icing sugar |
| DADP | Diacetone diperodixe |
| HTPB | Hydroxy terminated polybutadiene |
| SPEED | Shock physics explicit eulerian dynamics |
| STEC | Storage and transport of explosive committee |
| POP | Peak over pressure |
| HOF | Heat of formation |
| VOD | Velocity of detonation |
| AIT | Advanced imaging technology |
| HHMD | Hand-held metal detectors |
| DFMB | Door frame metal detectors |
| DSMD | Deep search mine detectors |
| ETD | Explosive trace detection |
| CT | Computed tomography |
| IMS | Ion mobility spectrometers |

This work is licensed under a Creative Commons Attribution 4.0 International License.









