Phytochemical Study and Antimicrobial Activity of Two Medicinal Plants from Al-Baha Region
Abdulaziz Ali Alomari, Abdalfatah Abdalla Fadlelmula* and Hassen Harzali
Department of Chemistry, Faculty of Science and Arts in Al-Mikhwah, University of Al-Baha, 65931 Saudi Arabia.
Corresponding Author Email: abdalfatah63@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350622
Article Received on : 31/08/2019
Article Accepted on : 02/12/2019
Article Published : 17 Dec 2019
The plant is a huge therapeutic source with enormous applications in curative industry. For new sources of antimicrobial agents, chloroform, ethyl acetate, and n–butanol extracts of two medicinal plants (Dodonaea viscose and Capparis spinosa) were prepared by liquid – liquid extraction. The plants were collected from Shuda mountain / Al-Baha region/ Saudi Arabia and then evaluated systematically. Phytochemical screening tests detect the existence of flavonoids, alkaloids, tannin, terponoids, saponnins and carbohydrates in most of the performed extracts. Antimicrobial activity was assessed against five bacterial and one fungal race. The extracts of the two plants leaves showed wide vision antibacterial activity and significant antifungal activity. The antibacterial activity (zone of inhibition) of Dodonaea viscose species varied from 30 to 18 mm, whereas of Capparis spinosa the range from 20 to 16 mm for ethyl acetate extracts, for n- butanol extracts the inhibition zone differ from 15 – 10 and 16 - 10 mm respectively. These results indicated that antimicrobial activities of plant species differ to a wide range not only between species themselves but also within the tests for antimicrobial evaluation. The current result supports the medicinal use of the leaves of these studied plants which works as an antimicrobial agent. These results compare to other studies carried out for the same plants in different countries in different environments exhibited diverse outcome in chemical constituents in the plant extracts and in their effects on tested types of micro organismswhich may have been due to a number of factors, including geographical location, season and environmental factors, and the method of extraction. This study for the two plant species was first time performed in this area of Saudi Arabia.
KEYWORDS:Capparis Spinosa; Dodonaea Viscose; Antimicrobial Activity; Phytochemical Screening. Zone Of Inhibition; Minimum Inhibition Concentration
Download this article as:| Copy the following to cite this article: Alomari A. A, Fadlelmula A. A, Harzali H. Phytochemical Study and Antimicrobial Activity of Two Medicinal Plants from Al-Baha Region. Orient J Chem 2019; 35(6). |
| Copy the following to cite this URL: Alomari A. A, Fadlelmula A. A, Harzali H. Phytochemical Study and Antimicrobial Activity of Two Medicinal Plants from Al-Baha Region. Orient J Chem 2019; 35(6). Available from: https://bit.ly/36Nr901 |
Introduction
Medicinal plants contain most ingredients in local medicines and most of Western prepared medicines comprise one or additional ingredients of plant origin [1]. Medicinal plants were used in folk medicine in various civilizations since ancient times due to its strong therapeutic effect on humans [2]. Medicines extracted from plants remain important sources, particularly in developing countries, to fight serious diseases. Approximately 60 to 80% of humans in the world still rely on folk remedies for the curing of popular diseases [3]. It is preferable to use manufactured medicines for several decades but now prefer to use herbal medicines to prevent sightless dependence on synthetic drugs. Latterly, plentiful plants have been assayed and notified for their power of the propagation of microorganisms, which can substituted the chemically manufactured medicines [2].
Dodonaea viscosa (Sapindaceae) is considered as evergreen Local plants in Saudi Arabia and it is spread in most warm countries [4]. Dodonaea viscosa has several therapeutic properties and is used as a folk remedy in many countries, it is given orally or as scumble to remedy many diseases [4]. In variety African and Asian countries, people prepared the yield of boiled dried leaves for curing of stomach ulcer after grinding and mixing them with milk or honey, and is involved in the therapy of hemorrhoids and stomach pain concerning to the liver and spleen [4]. Moreover to their traditional uses, several surveys have proved their therapeutic value, as they confirm their anti-inflammatory effect, anti-oxidation and hypolipidaemic effect [4].
Capparis species pertinence to family Capparaceae is not an exception to therapeutic properties. This family comprise of 250 species, including C. spinosa and C. decidua are domestic to Saudi Arabia which has been reported for their uses in traditional medicine in humans (Mabberley, 1997; Hamed et al., 2007). Upper parts of these sorts have been notified to be effective against bacteria. The extract of the fermented plant parts have been conveyed to be effective to preventing the evaluation of diverse bacterial strains, especially those that show resistance to medications such as ciprofloxacin, vancomycin and teicoplanin [2].
The studies in the field of medicines exhibit that Dodonaea viscosa hold over antidiabetic, antimicrobial, insecticidal, antioxidant, cytotoxic, antifertility, anti-inflammatory, analgesic, anti- ulcer, antispasmodic, anti-diarrheal and detoxification influence [5].
Scientifically, the phytochemical researches of capers extracts offered the presence of numerous chemical classes with very interesting biological activities. Ingredients present are alkaloids, fatty acids, phenolic acids, flavonoids, aldehydes, esters, vitamins and glucosinolates. Other studies by Ali-Shtayeh & Abu-Ghdeib (1999) established that the water extract of Capparis spinosa has antifungal characteristics together with a percentage of suppression greater than 90% against T. violaceum which in charge of ringworm and which is wide difuce in Africa and Asia [6].
The targets of this investigation were to evaluate the role of ethanol. chloroform, ethyl acetate, and n-butanol extracts of leaves of D. viscosa and C. spinosa from Jebal Shuda / Al-Baha area for potential antimicrobial effect, against human infective bacteria, yeast (Candida albicans), and plant infective bacteria, and also, study the (MIC), and (MBC) of the plant extracts, and to trial the sensitivity of infective microorganisms for antibiotics, and compared it with the effect of the plant extracts.
Materials and Methods
Material
The plant samples leaves ( Dodonaea viscose and Capparis spinosa) were gathered from Shuda mountain in Almakhwah region on march 2018 were classified, confirmed by Biology department, a voucher samples were kept in Biology department College of Sciences and Arts / Almakhwah, Al-Baha University.
Preparation of the ethanol extract
The leaves samples of the two plants were cleaned dried in shade for one month at room temperature and powdered. The powdered materials (500 g) were macerated in 3 liters of 80% ethanol and left for three days at room temperature.The samples then filtered and the collected filtrates were concentrated with rotary evaporator, after that the extracts were dried at room temperature for 10 days. Eventually the extracts were kept in vacuum desiccators for three days for complete dryness.
Fractionation of the ethyl alcohol extract through liquid – liquid extraction
Each ethyl alcohol extract was dissolved in 200 mL of distilled water and then was transferred to separotary funnel, subsequently it was extracted consecutively with the organic solvents in order of increasing polarities initiated with petroleum ether, then chloroform, ethyl acetate and n– butanol. The organic layers were separated and concentrated, with rotary evaporator and then drain off at room temperature for ten days.
Screening of Phytochemical Components
The leaves extracts were subdued to specific tests using the standard methods for the establishment of the chemical components [7 – 8].
Test for Phenolics
The extract (0.25g) was resolved in 10 mL of water and then refined. A small drops of 0.1% ferric chloride were appended to the filtrate. The occurrence of strong brownish-green or a blue- black color represents the existence of phenolics in the samples.
Test of tannins
The dried ground sample (0.5 g) was boiled in 15 mL distilled water in small beaker and filtered. A little quantity of 0.1% ferric chloride was added. The occurrence of brownish green or blue-black colour proven the existence of tannin in the sample.
Test of steroids
Acetic anhydride (2 mL) was appended to 0.5 g ethanolic extractor of each specimen with 2 mL sulphuric acid. Changes of the tint from purple to blue or green represent the occurrence of steroids.
Determination of terpenoids
The extract (5 mL) of each extract was added to a carefully prepared mixture of 1 ml chloroform and 1 mL concentrated sulphuric acid. The appearance of a reddish brown colour in the boundary emphasized the existence of terpenoids.
Test of anthraquinones
Plant powdered sample (1 g) was heated to boil with 10 mL of 0.5 M KOH including 1 ml of 3% H2O2 solution. The blend was boiled, cooled, filtered, and to 5 mL of filtrate few quantity of glacial acetic acid was added. The acidified blend was motivated with 10 mL of benzene and allowed to stand. A 5 mL of the benzene solution was mobilized with 3 mL of 10% ammonium hydroxide solution and the benzene layer was left to stand. The presence of anthraquinones was indicated by pink or red colour.
Test of flavonoids
To 3ml of the filtrate, 0.5 mL of concentrated hydrochloric acid was inserted, followed by appending of few magnesium ribbons. Appearance of pink trint represents the occurrence of flavonoids.
Test of saponnin
The ground specimens (2.0 g) was mixed with 20 mL of distilled water and heated in water bath and filtered. Ten ml of the filtrate was blended with 5.0 mL of distilled water and stirred powerfully for stable permanent foam. Few drops of olive oil were added to the foam and the blend was strongly shaken to obtain an emulsion.
Antimicrobial Assay
Bacteria such as Staphylococcus aureus, Bacillus subtilis, Streptococcus pyogenes, Pseudomonas auroginosa, Klebsiellapnemonia,and Fungi (Candida albicans) were used in this investigation. Antimicrobial activity of the previous considered extracts were run using the agar diffusion method depicted by Collins and Lyne, (1970). All the above remained bacteria were inoculated into nutrient agar medium and fungi inoculated to potato dextrose agar medium. The well of 8 mm diameter was digged in the culture medium using sterile cork borer. The collected extracts were intensified and filled into sterile readymade discs (Hi-media, Bombay) in various volumes of 6.25, 12.25, 25, 50, and 100 μl / disc respectively and left to dry for one a day at 25 oC. The
agar plates were expand with 100μl of actively growing broth cultures of the special bacteria and are left to drain off for 10 min. The sterile available discs filled with each extract distributively (6.25, 12.25, 25, 50, and 100 μl / disc respectively) were enjoined on the vaccinated plates. Different extracts were run to capacity in each well. Culture plates were brooded at 37oC for one day in bacteria and incubated at 37oC for 4 days in fungi. Bioactivity was specified by scale
diameter of inhibition zones in mm. Solvents used for extraction render as control [9].
Statistical Analysis
Statistical comparisons were carried out by one- sample t- test analysis using SPSS Statistics version 16. Differences were significant at the level of p ≤ 0.05. All the data are reported as means ±standard deviation (SD) of triplicate determinations (n= 3).
Result and discussion
The chemical analysis
Tests for active ingredients were performed on all the studied leave extracts of Dodonaea viscose and Capparis spinosa, the results were reported in Table 1. Which explained that the leaves extracts of D. viscose of all fractions (petroleum ether, chloroform, ethyl acetate, n-butanol and ethanol) exhibited the occurrence of phenolics, flavonoids, tannins, terpinoid, and carbohydrate while the ethyl acetate and n-butanol fraction showed the absence of the alkaloids. Saponin was missed in petroleum ether fraction. Furthermore in Table 2 (leaves extracts of C.spinosa), falvonoids, tannin, and carbohydrate were occured in n-butanol, ethyl acetate, chloroform and ethanol fractions. Flavonoid, tannin, saponin, and carbohydrate were not detected in petroleum ether extract of C. spinosa. These results differ from those obtained by (Riaz et al; 2012) whose revealed the presence of tannin only in ethyl acetate and n – butanol in Dodonaea viscose leave extracts [1], whereas in this study tannin was present in all fractions but the two studies agreed upon the presence of terpinoid in all fractions.( El-Sawi et al; 2018) confermed that alkaloid was absent in the leave extract of D. viscose whereas flavonoid, tannin, saponin, steroid and carbohydrate were present in moderate concentration [15]. whilst in this study all classes of natural products were present in moderate to high concentrations. On the other hand (Ramamurthy et al; 2018) their results came in accord with result of this study[9].
(Tagnaout et al 2016). studied the chemical constituents of Capparis spinosa leaf extracts in different solvents [6]. The results obtained by them were considerably different from what we found. They revealed the absence of tannin and saponin in the plant extract however we found them in our study particularly in ethyl acetate, n- butanol and ethanol extracts. At the same time (AL-Azawi et al 2018), obtained the same results as we found in our study in all extracts of C. spinosa [16]. This reflect the effect of the climate in the chemical constituents and in their concentrations in the plants that grow wildly in different enviroments. Also represent the influence of the method of the extraction in the same aspect.
Table 1: Results of chemical analysis of Dodonaea viscose leave extracts
|
Extract |
Flavonoid | Tannin | Alkaloid | Terpinoid | Saponnin | Carbohydrate |
| Petroleum ether | + | + | + | + | – |
+ |
|
Chloroform |
++ | ++ | ++ | ++ | – | + |
| Ethylacetate | +++ | +++ | – | ++ | +++ |
++ |
|
n-butanol |
+++ | ++ | – | + | ++ | +++ |
| Ethanol | ++ | +++ | ++ | + | ++ |
++ |
+ = positive, ++ = good present, +++= strongly present, – = not detected
Table 2: Results of chemical analysis of Capparis spinosa leave extracts
|
Extract |
Flavonoid | Tannin | Alkaloid | Saponnin | Carbohydrate |
| Petroleum ether | – | – | + | – |
– |
|
Chloroform |
+ | + | ++ | – | + |
| Ethylacetate | +++ | ++ | + | ++ |
+ |
|
n-butanol |
+++ | ++ | – | + | +++ |
| Ethanol | + | + | – | + |
++ |
+ = positive, ++ = good present, +++= strongly present, – = not detected
The results in Table 1 and 2 exhibited that Dodonaea viscose extracts were rich with Flavonoids and phenolic compounds than that detected in the homologous extractors of C. spinosa, which mean that D. viscose was very rich of antioxidants compounds and is an excellent source for these compounds. These results supported and confirmed the utilize of these medicinal plants as traditional medicine for remedy of many ailments.
Antimicrobial Activity
Maximum zone of inhibition for 100 mg/mL was in recorded by the ethyl acetate extract of Dodonaea viscose leave was 30 mm against Bacillus subtilis. Similarly 20 mm were registered by ethyl acetate of Capparis spinosa leave extract was observed against Bacillus subtilis. Chloroform extract of the two species showed no efficacy against all test bacteria organisms except Bacillus subtilis (15 mm). Ethyl acetate and n-butanol extracts exhibited high to moderate activity 10-30 mm against all test bacteria organisms (Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus agalactiae) by the leaves extracts of both species (Table 3 and 4).Salama et al 2018 reprted that Dodonaea viscose leave extracts showed moderate to low activity against various tested pathogens ( zone of inhibition in the range 7 – 10 mm) whereas we found strong antibacterial activity in our study(30 – 9 mm) In this search, Staphylococcus aureus showed higher response for the extracts of D. viscose except chloroform extract and this report is similar to that of (Udaya prakash et al 2012) [17].But the record result for Bacillus subtilis is differ, as Udaya prakash et al found that B. subtilis was affected with all extracts we found n- butanol extract not influenced. More over AL-Azawi et al [16] obtained different results, they reported that, Staphylococcus aureus and Escherichia coli reflected high response to C. spinosa leave extracts whereas Pseudomonas aeruginosa was not affected but we found Staphylococcus aureus and Pseudomonas aeruginosa showed high to moderate response respectively whilst Escherichia coli exhibit moderate response. This may be due to the occurrence of terpenes, phenols, flavonoids, saponins, in n–butanol and ethyl acetate extracts, which have a great effect as antimicrobial agents (Table1 and 2) [10]. Ethyl acetate and n– butanol leaves extractors of D.viscose and C. spinosa showed moderate effect against fungi C. albicans, the zone of inhibitions for 100 mg/mL were (18, 10 and 17, 10 mm respectively).
Escherichia coli showed good susceptibility towards the ethyl acetate and n–butanol leave extracts of Dodonaea viscose, where the MIC were 25, and 12.25 mg/mL respectively and for the Capparis spinosa, the MIC were 25, 25 mg/mL (Table 3, 4). These extracts for the two plant species have a pronounced effect on the outgrowth of S. aureus, B. s subtilis and C. albicans, the MIC are 6.25, 12.5, 25, 25, 12.5, and 50 for Dodonaea viscose and are 25, 25, 25, 50, 50, 25, and 25, 50 mg/mL for Capparis spinosa (Table 5, 6). These results supported the work that has been summarized by Rojas et al., 1992; Mothana et al. who announced that the ethyl acetate extracts of D. viscose and Capparis spinosa have antimicrobial effects [11, 12]. The response of the five microorganisms (Staphylococcus aureus, Bacillus subtilis, Streptococcus pyogenes, Pseudomonas auroginosa, Klebsiellapnemonia,and Candida albicans) towards Dodonaea viscose leave extracts were represented in Fig.1
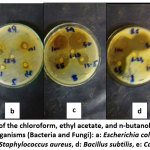 |
Figure 1: The effect of the chloroform, ethyl acetate, and n-butanol leaves extracts of D. viscose on test organisms (Bacteria and Fungi): a: Escherichia coli, b: Pseudomonas aeruginosa, c: Staphylococcus aureus, d: Bacillus subtilis, e: Candida albicans. Click here to View Figure |
Table 3: Results of zone of inhibition (mm) of Dodonaea viscose leave extracts
| Extract | Extract volume µl /disc | Microorganism | ||||||
| Ec | Pa | Sa | Bs | Ca | ||||
| Chloroform | 100 | Diameter of inhibition zone (mm) | ||||||
| – | – | – | 14.9 ± 0.14 | – | ||||
| 50 | – | – | – | 14.3 ± 0.49 | – | |||
| 25 | – | – | – | 10.1 ± 0.21 | – | |||
| 12.50 | – | – | – | – | – | |||
| 6.25 | – | – | – | – | – | |||
| Ethyl acetate | 100 | 24.8 ± 0.25 | – | 20.4 ± 0.63 | 30.4 ± 0.63 | 18.4 ± 63 | ||
| 50 | 18.2 ±0. 28 | – | 20.1 ± 0.21 | 20.3 ± 0.49 | 17.7± 0.35 | |||
| 25 | 9.8 ± 0.28 | – | 15.3 ± 0.42 | 20.3 ±0.42 |
15.1 ± 0.14 |
|||
| 12.50 | – | – | 15.4 ± 0.40 | – | 14.7 ± 0.42 | |||
| 6.25 | – | – | 9.9 ± 0.35 | – | – | |||
| n- Butanol | 100 | 14.9 ± 0.14 | 13.9 ±0.07 | 9.8 ± 0.28 | – | 10.2 ± 0.35 | ||
| 50 | 13.1 ± 0.14 | 10.2 ± 0.35 | 10.1 ± 0.14 | – |
9.8 ± 0.28 |
|||
| 25 | 9.5 ± 0.70 | 10.2 ± 0.28 | 10.4 ± 56 | – | – | |||
| 12.50 | 9.9 ± 1.27 | 9.5 ± 0.70 | 10.3 ± 0.49 | – | – | |||
| 6.25 | – | – | – | – | ||||
Ec: Escherichia coli, Pa: Pseudomonas aeruginosa, Sa: Staphylococcus aureus, Bs: Bacillus subtilis, Ca: Candida albicans, and – : no inhibition zone. The results were reported as mean ± SD (n =3).
Table 4: Results of zone of inhibition (mm) of Capparis spinosa leave extracts
| Extract | Extract volume µl /disc | Microorganism | ||||
| Ec | Pa Sa Bs | Ca | ||||
| Chloroform | 100 | Diameter of inhibition zone (mm) | ||||
| – | – | – | 13.3 ± 0.42 | – | ||
| 50 | – | – | – | 10.3 ± 0.49 | – | |
| 25 | – | – | – | – | – | |
| 12.50 | – | – | – | – | – | |
| 6.25 | – | – | – | – | – | |
| Ethyl acetate | 100 | 18.7 ± 0.49 | 18.1 ± 0.07 | 16.1 ± 0.14 | 19.8 ± 0.28 | 16.8 ± 0.21 |
| 50 | 16.9 ±0. 07 | 15.9 ± 0.14 | 14.6 ± 0.84 | 18.2 ± 0.21 | 13.5± 0.70 | |
| 25 | 14.9 ± 0.14 | 10.1 ±0.14 | 9.8 ± 0.28 | 15.3 ±0.35 | 10.2 ± 0.28 | |
| 12.50 | – | – | – | – | – | |
| 6.25 | – | – | – | – | – | |
| n- Butanol | 100 | 16.3 ± 0.35 | 14.9 ±0.07 | 10.1 ± 0.21 | – | 9.9 ± 0.14 |
| 50 | 12.9 ± 0.07 | 12.1 ± 0.14 | 9.1 ± 0.07 | – | 10 ± 0.00 | |
| 25 | 9.9 ± 0.21 | 10.1 ± 0.07 | – | – | – | |
| 12.50 | – | 9.9 ± 0.14 | – | – | – | |
| 6.25 | – | – | – | – | – | |
Table 5: Results of Minimum Inhibitory Concentration (MIC) (mg/mL) of Dodonaea viscose leave extracts
|
Extract |
Concentration of extractin mg /ml | Microorganism | ||||||
|
Ec Pa Sa Bs Ca |
||||||||
| Chloroform |
100 50 25 |
— | — | (MIC) (mg/ml)– |
– – |
|||
| 12.506.25 | — | — | — | 25 | — | |||
| Ethyl acetate | 1005025
12.50 6.25 |
25 | —
– – |
6.25 | 25 |
12.50 |
||
|
n- Butanol |
1005025 | 12.50 | 12.50 | 12.50 | — | 50 | ||
| 12.506.25 | – | |||||||
Table 6: Results of Minimum Inhibitory Concentration (MIC) (mg/mL) of Capparis spinosa leave extracts
| Extract | Concentration ofextract in mg / ml | Microorganism | ||||||
| Ec | Pa | Sa | Bs | Ca | ||||
| Chloroform | 100 | (MIC) (mg/ml) | ||||||
| – | – | – | 25 | – | ||||
| 50 | – | – | – |
– |
||||
| 25 | – | – | – | – | ||||
| 12.50 | – | – | – |
– |
||||
| 6.25 | – | – | – |
– |
||||
| Ethyl acetate | 1005025
12.50 6.25 |
25 | 25 | 25 | 25 | 25 | ||
| n- Butanol | 100 | 25 | 12.50 | 50 | – | 50 | ||
| 50 | – | |||||||
| 25 | – | |||||||
| 12.50 | – | |||||||
| 6.25 | – | |||||||
Antimcrobial activity versus Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtilis, and Candida albicans exhibied that the plant can be used in the curing of gastrointestinal infection and diarrhea in human. This may be referred to its containment in phytochemical components like alkaloids, flavonoids, saponins, tannins, amino acids and carbohydrates, which were also reported by Ikewuchi et al.,
Conclusion
The findings of this study suggest the importance of D. viscose and C. spinosa L species for use in pharmacy and phytotherapy.Actually, phytochemical screening reveals the richness of the plants by secondary metabolites that have alimentary and medicinal important for human being healthy because most of the plants extracts constitute the active ingredients such as alkaloids, flavnoids, terponoids, steroids, saponins, and tannin. Moreover, D. viscose and C. spinosa L can constitute an interesting source of natural antioxidants towards the free radicals in comparison with other provenances. In the present research, MIC of the extracts showed relatively best activities in all the tests obviously caused by effective extraction of phytochemicals. It is concluded on this study that n-butanol, and ethyl acetate extracts (leaves of D.viscose and C. spinosa) from Jabal Shuda / South Saudi Arabia have obvious effect on the tested microorganisms, and these results as compared with other studies, indicated that the type of solvents and procedure of extraction of D. viscosa and Capparis spinosa significantly affects the quantity of the chemical components, thus some antimicrobial effects differ from that reported in the literature.
Acknowledgments
The authors would like to thank University of Al-Baha for providing financial support to this project.
Conflicts of Interest
The authors announce that there are no conflicts of interest related to this article.
References
- Riaz, T.; Abbasi, M. A.; Aziz-ur-Rehman, S. T.; Ajaib, M.; Khan, K. M. Serb. Chem. Soc., 2012, 77(4), 423–435. Doi:10.2298/JSC11061183R
- Gull, T.; Sultana, B.; Bhatti, I. A.; Jamil, A. J. Agric. Biol., 2015, 1814–9596. Doi:1017957/IJAB/14.0007
- Ramzi, A.; Mothana, A.; Salah, A.; Abdo, A.; Sidgi, H.; Faisal, M.; Althawab, N.; Sama, A.; Alaghbari, Z.; Ulrike, L. Acc. Pub., 2010, 7(3), 323–330. Doi:10.1093/ecam/nen004
- Shafek, R. E.; Shafik, N.H.; Michael, H. N.; El- Hagrassi, A. M.; Osman, A. F. Chem. Pharm. Res., 2015, 7(5),109-116.
- Al-Snafi, A. E. IOSR J. Pharm; 2017, 7(2), 10-21. Doi:10.9790/3013- 0702011021
- Tagnaout, I.; Zerkani, H.; Mahjoubi, M.; Bourakhouadar, M.; Alistiqsa, F.; Bouzoubaa, A.; Zair, T. Pharmacogn. Phytochem., 2016, 8(12), 1993-200.
- Harborne, J. B., 3rd Edition, Chapman and Hall Ltd., London., 1973, 49-188.
- Zohra, S. F.; Meriem, B.; Samira, S.; Muneer, M.S.A. J. Nat. Prod. Plant Resour; 2012, 2(4), 512-516.
- Rama Murthy,V.; Maria Rajeswari, D; Gowri, M. K.; Jayanthi, G.; raveendran, S. J. Pure Appl. Zool., 2013,1(2), 178-184.
- Ekwenye, U. N.; Elegalam, N. N. Mol. Adv Sci., 2005, 1(4), 411-416.
- Rojas, A.; Hernandez, L.; Pereda, M. R.; Mata, R. Ethnopharmacology., 1992, 35(3), 275-283. Doi:10.1093/ecam/nen004
- Mothana, R. A. A.; Abdo, S. A. A.; Hasson, S.; Althawab, F. M. N.; Alaghbari, S. A. Z.;Lindequist, U., 2008.
- Moideen, M. M. J.; Raffick, M. M. J. Phytopharmacology., 2012, 3(1), 21- 26.
- Ikewuchi, C. C.; Ikewuchi, C.J.; Ayalegy, O.E.; Onyeike, N.E. Appl. Sci. Environ Manage., 2010,14, 103-106.
- El-Sawi. S. A; Motawe. H. M; Ahmad.S. S; Ibrahim. M. E. Mater. Environ. Sci., 2018, 9 (5), 1495-1502. Doi.org/10.26872/jmes.2018.9.5.164
- AL-Azawi.A. H., Ghaima.K. K., Salih. H. H. Bioscience Research., 2018, 15(3), 2611-2618. online freely at www.isisn.org
- Udaya prakash, N.K.; Selvi. C.R.; Sasikala,V.; Dhanalakshmi S.; Bhuvaneswari udaya, P.S. International Journal of Pharmacy and Pharmaceutical Sciences., 2012, 4, (2), 509-512

This work is licensed under a Creative Commons Attribution 4.0 International License.









