Thermodynamic Studies of the Removal of Lead from Synthetic Wastewater Using Cyperus Rotundus
Davoud Balarak1, Hadise Mardanshahi2, Kethineni Chandrika3* and C.S.Felice4
and C.S.Felice4
1Department of Environmental Health, Health Promotion Research Center, Zahedan University of Medical Sciences, Zahedan, Iran
2Student Research Committee, Zahedan University of Medical Sciences, Zahedan, Iran.
3Department of Petroleum Engineering, KoneruLakshmaiah Education Foundation, Vaddeswaram, Guntur, AP, India–52250
4Department of Biotechnology, KoneruLakshmaiah Education Foundation, Vaddeswaram, Guntur, AP, India–52250
Corresponding Author E-mail: kkchandrika@kluniversity.in
DOI : http://dx.doi.org/10.13005/ojc/350508
Article Received on : 28-07-19
Article Accepted on : 03-10-2019
Article Published : 26 Oct 2019
This research wasaccomplished to appraise the performance of Cyperus Rotundus Stalk (CRS) in adsorption of lead heavy metalofaquatic environments. For this purpose, the batch system was used for review the effect pH, Mixing time, adsorbent particle size and temperature for Pb(II) removal on the CRS. The highest lead removal efficacy was achieved in pH=6 and it was considered as the optimum pH. The result indicated the maximum Pb(II) adsorption at the contact time of 90 min which implies that increase in contact time lead to a higher lead uptake.The amount of R2using the pseudo-second-order are greater than compare with others models.Influence of temperature was determined byusing thermodynamic parameters and the results showedremoval of lead on the CRS was endothermic, spontaneousandfeasible.
KEYWORDS:Pb(II); Cyperus rotundus stalk (Nut grass); Thermodynamics, Kinetics; Synthetic Wastewater
Download this article as:| Copy the following to cite this article: Balarak D, Mardanshahi H, Chandrika K, Felice C. S. Thermodynamic Studies of the Removal of Lead from Synthetic Wastewater Using Cyperus Rotundus. Orient J Chem 2019;35(5). |
| Copy the following to cite this URL: Balarak D, Mardanshahi H, Chandrika K, Felice C. S. Thermodynamic Studies of the Removal of Lead from Synthetic Wastewater Using Cyperus Rotundus. Orient J Chem 2019;35(5). Available from: https://bit.ly/2pOxqs1 |
Introduction
Heavy metals are most important pollutants and its presence in wastewaters can be the striking environmental issue,after all, dissolved toxic metal ions can finally enter the food chain and thus become for human health is a risk factor.1, 2. A number of industries, such as metalworking, mining and leather tanning, have a significant role in releasing of the heavy metals into the effluents; this event concludes to the freshwater pollutionand receiving waters3. Heavy metals are not biodegradable and can accumulate in tissues and can create different diseases and disorders4.
One of such heavy metal of concern is Pb(II) which the inadvertent exposure and consumption of the water and food contaminated by this element for extended periods, even with its low concentration, can bring an extensive range of health issues, e.g., cancer, convulsions, nausea, subtle effects on metabolism and intelligence, renal failure, nausea , coma 5, 6. Over the years, more than a few methods have progressed to remove the lead existed in effluent7. At higher concentrations, lead impairs cognitive development, and introduced as an enzyme inhibitor in the body, because it can replace the vital element zinc from heme enzymes.8. The highest allowable limit of Pb(II) in potable water which is publicized by the World Health Organization is 50 g/L, while the United States Environmental Protection Agency has approved the values lesser than 15 g/L as the safe limit in this case9. The above-mentioned effects of the lead demonstrate the obligation of removing these elements from waters and wastewaters to enhance the protection of public health and environment10.
Although, there are various methods to eradicate the heavy metal such as adsorption,reverse osmosis,filtration, ion exchange, electro chemical andprecipitation 1; however, nearly all of them have not an adequate performance comparing to their operational cost12. Precipitation methods has found to be an authentic method but they need to large settling tanks and a subsequent treatment which it is led to diminish the applicability of this method13, 14. Ion exchange is another method in field of the removing the heavy metal,which allows the extraction of metal ions, but it is an expensive and complex method.15.
Adsorption has classified as an economically sensible substitute technique to remove the lead. Different materials as absorbent have been appliedfor remove heavy metal from solutions such as Azolla filiculoides, canola, Lemna minor, corn husk ash, Orange peel, and banana.16-19. Nowadays, this type of material is increasingly attracting attention to be used to remove heavy metals.
Cyperus Rotundus has recognized as one of the most invasive weeds which it can extensively observed in tropical and temperate regions 20. It can be found in more than 90 countries and can infest more than 50 crops; hence, it is called as the world’s worst weed 21.Therefore, CRS was appliedfor decrease lead as an important contaminant from an aquatic environment at adsorption process.The effect of influential parameters atbatch adsorption process such as mixing time, CRS Particle Size, temperature and pH was investigated.
Experimental
Preparation of Adsorbent
The Cyperus Rotundus Stalk was gatheredfrom agricultural university of Tabriz. The CRS was first washed carefully with deionized water. Then, they rinsed with the hot deionized water (75◦C) were used to remove the soluble colored. The dried biomass sample was exposedat 0.5 M HCL solution for 120 min under slow stirring. The CRS was dried at 75 ◦C at 24 h. The dried CRS werechopped and siftedat 10 to 100 mesh and stored in polythene bottles.
Preparation of Pb(II)solution
A stock solution of lead (concentration of 1000 ppm) was provided using an accurately weighed quantity of the Pb(NO3)2 in double-distilled water. Various concentration of lead solution provided with diluted the stock solution. 0.1 N NaOH and HCL were used to adapt the pH values of metal solutions.
Batch adsorption experiments
All chemicals used were provided by Merck (USA). These chemicals are analytical reagents and used without further purification. All solutions wereprovidedby the double-distilled water. To conduct a batch experiment, 0.1 L of lead solution (concentrations of 10–200 ppm) were poured into a containers (200 ml), then 2.5 g/L or 0.25 g/100 of CRS was added to the solutioncontaining lead. Finally, the prepared mixture is stirred for 1.5 hours at 150 rpm on a mechanical shaker and passed 0.45 μm nitrocellulose membrane. To measure the leadsolution, the AAS (Atomic Absorption Spectrophotometer) were utilized. The experiments in present study were repeated 3 times and the average of these three step experiments were presented as the results. It should be noted that the control experiments were performed using the blanks containing no metal ions.
The below equations are applied to determine the %removal and amount adsorbed (mg/g)of lead: 22-24
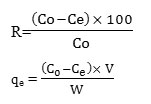
C0 is the lead concentration at initial solution (mg/L), Ce is the concentration of lead in effluent after removal (mg/L), V is the amount of the solution (L) and W is the weight (g) of the CRS applied.
Results and Discussion
Effect of CRS Particle Size the Adsorption of lead
Fig. 1 epitomizes the leadremovalby CRS at different adsorbent particles size. This Fig indicates that the increasing of particle size is led to reducing the adsorption capacity. The highest lead adsorption of CRS recorded was 71.60 mg/g which it was related to a particle size of 100 mesh. This event observed in this part of the study can be elucidated by the topic that the fine size of the CRS particles has the greater interior surface area and the micro-pore amount which it can be concluded to more active sites for adsorption25-27. But, for higher particles, the pore diffusion resistance is greater for mass transfer, which it can decrease and can eliminate the utilization of the internal surfaces of the particle in adsorption of the Pb(II) and it can consequently decrease the amount of Pb(II) adsorbed28, 29.
Effect of PH on Lead Removal
The pH has identified as a very important parameter that can significantly affect the biosorption process. The optimization of pH in the present study was performed at the pH between of 2.0–8.0. Data obtained of this section is presented in Fig. 2. As it can be observed, The Pb(II) removal is absolutely dependent upon the pH up to 6.0 and it remains practically constant after this pH value,and the %removal was 89.9% at this pH. The competition raised between the lead ions and H+ ions which it exists on the binding sites of the cells are the reason to decreasing the adsorption capacity at lower pH 30, 31.
Determination of thermodynamic parameters
The effect of the temperature was evaluated at various temperatures (25, 35, 45 and 55 °C) and the data obtained of this section were presented in Fig. 3. The higher temperature assists to advance the removal of lead on CRS (i.e. endothermic process). Furthermore, it may produce a inflation effect within the internal part of the biosorbent which it can help to penetrate more Pb(II) ions32, 33.
Thermodynamic parameters, i.e., free energy change ( G◦), enthalpy change ( H◦), and entropy change ( S◦) is utilized to determine whether the adsorption process is spontaneous or not. G◦ is determined by the below equation34-36.
ΔG◦ = -RT Ln K
R and T are the universal gas constant and temperature, and K is the distribution coefficient. The following equation expresses the relation between ΔG◦, ΔH◦ and ΔS◦ 37, 38:
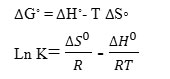
The amount of ΔG◦, ΔH◦, and ΔS◦ related to the removal of lead on the CRS at various temperatures asshown in Table 1. Negative amount of ΔG◦ obtained shows the possibility of the adsorption process. The positive amount of ΔH◦ proves the endothermic nature of adsorption. The positive amount of ΔS◦ implies increase randomness at the solid/solution interface during the removallead on the CRS.
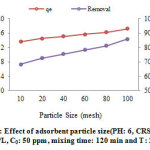 |
Figure 1: Effect of adsorbent particle size(PH: 6, CRS dosage: 2.5 g/L, C0: 50 ppm, mixing time: 120 min and T: 25 ) Click here to view figure |
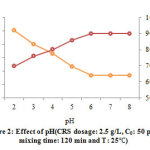 |
Figure 2: Effect of pH(CRS dosage: 2.5 g/L, C0: 50 ppm, mixing time: 120 min and T: 25 ) Click here to view figure |
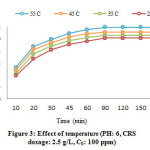 |
Figure 3: Effect of temperature (PH: 6, CRS dosage: 2.5 g/L, C0: 100 ppm) Click here to view figure |
Table 1: Thermodynamic data for lead removal onto CRS at various temperatures
| T (◦C) | G◦ (kJ/mol) | S◦ (J/mol K) | H◦ (kJ/mol) |
| 25 | -1.044 | 34.48 | 7.291 |
| 35 | -1.95 | ||
| 45 | -2.36 | ||
| 55 | -2.87 |
Effect of Adsorbent Dose
The effect of CRS dosage on the removal of lead was applied by changes the mass of CRS in the value 0.5-4 g/L (Fig 4). The adsorption capacity leaned to decrease with the increase of CRSamount. At higher dose of adsorbent, the adsorbent may aggregate which it can reduce the number of binding site and consequently, the adsorption capacities 39.
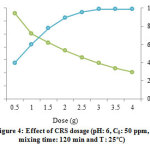 |
Figure 4: Effect of CRS dosage (pH: 6, C0: 50 ppm,mixing time: 120 min and T: 25 ) Click here to view figure |
Kinetic models
Kinetic studies are important for any type of adsorption process.For the adsorption model, Pseudo-first-order (PFO) and pseudo-second order (PSO) kinetic and Intra-particle diffusion (IPD) can be proposed. PFO kinetics is to description the rate of adsorption process in liquid-solid phase. The PFO is represented as 40:
![]()
The above equationafter definite integration41, 42:
![]()
The PSO equation can be shown following Eq43, 44:
![]()
Where, K1 and K2 are the rate constants of the PFO and PSO.
The linear form of the above-mentioned Eq is 45:
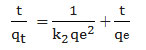
The findingsare given in Table 2. The amount of R2 with PFO wasless of 0.912, while for PSO was high of 0.99 for all concentrations. It is comprehensible that the removal of lead on CRS biomass is better demonstrated by PSO which it indicates the adsorption system pertain to the PSOmodel.
IPD is another model which was also utilized to realize whether the rate restrictive step is the IPD, film diffusion, or mass action. Since the mass action is a extremely quick step in physical adsorption; hence it can be neglected. Weber-Moris represented the IPD model and it can be written following Eq45:
qt = Kd t1/2 + I
The value of the slope correlates to IPD constant and the intercept value at an approximate value of the boundary layer thickness (Fig 5). The data at three different concentrations illustrate two stages of linearity. The first stage is related to the immediate adsorption which it completed well within the initial 45 min; the gradual adsorption is the second stage. Both the linear lines, which they are not passing through the origin, suggest that there are other limiting mechanisms in adsorption process and the IPD is not the only limiting mechanism. Table 2 represents the kd and R2 obtained for the IPD. The values of R2 are lower than expected values of the PSO model which it is clarify that the qexp values are not in accordance with the IPD.
Table 2: Kinetic data for the removal of lead on CRS biomass at various concentration
|
Co (mg/L) |
qe exp (mg/g)
|
PFO | PSO | IPD | ||||||
| K1 | qe cal | R2 | K2 | qe cal | R2 | Kd | I |
R2 |
||
|
50 100 200 |
14.2929.5548.18 | 0.03540.04850.0562 | 8.49121.7332.65 | 0.8890.9120.858 | 0.08240.05960.0312 | 11.5627.4446.39 | 0.9980.9960.993 | 0.1480.1910.236 | 0.8491.6541.974 |
0.825 0.794 0.841 |
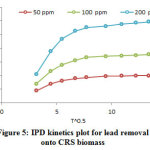 |
Figure 5: IPD kinetics plot for lead removal onto CRS biomass Click here to view figure |
Conclusion
The results showed that the CRS biomass is a flourishing adsorbent towards the adsorption of heavy metal lead from aquatic environments. The pH was found to be an effective parameter on leadremoval, so that the increasing of pH is led to develop the removal efficacy. pH=6 was observed to be the optimum pH. Thermodynamic studies showed that a viable and impulsive and endothermic process occurs during the removal of lead on CRS. It is also confirmed by this fact that the leadadsorption increases by temperature rise.
Acknowledgements
The authors are grateful to the Zahedan University of Medical Sciences for the financial support of this study.
Conflicting interest
There is no conflicting interest in this study.
References
- Mohan, D.; Pittman, D. C. U.; Bricka, M.; Smith, F.; Yancey, B.; Mohammad, J. P. J. Colloid. Interface. Sci. 2007, 310, 57–73.
- Shin, E. W.; Rowell, R. M. Chemosphere. 2005, 60, 1054–1061.
- Balarak, D.; Azarpira, H. Int. J. ChemTech. Res. 2016, 9(9), 543-549.
- Balarak, D.; Joghataei, A.; Azarpira, H.; Mostafapour, F. K. Int. J. Pharm. Technol. 2016, 8(3), 15780-88
- Bulut, Y.; Tez, Z. J. Hazard. Mater. 2007, 149, 35–41.
- Axtell, N. R.; Sternberg, S. P. K.; Claussen, K. Bioresour. Technol. 2003, 89, 41–48.
- Amarasinghe, W. P. K.; Williams, R. A. J. Chem. Eng. 2007, 32, 299–309.
- Yurtsever, M.; Sengil, I. A. J. Hazard. Mater. 2009, 163, 58–64.
- Soylak, M.; Elci, L.; Akkaya, Y.; Dogan, M. Anal. Lett. 2002, 35, 487–499.
- Balarak, D.; Azarpira, H. Int. J. ChemTech. Res. 2016, 9(7), 566-573.
- Eren, E.; Afsin, B.; Onal, Y. J. Hazard. Mater. 2009, 161, 677–85.
- Azarpira, H.; Mahdavi, Y. Pharma. Chem. 2016, 8(12), 61-67.
- Balarak, D.; Azarpira, H.;Mostafapour, F. K. Pharma. Chem. 2016, 8(10), 243-247.
- Yin, P.; Yu, Q.; Lin, Z.; Kaewsarn, P. Environ. Technol. 2001, 225, 509-614.
- Ramesh, S. T.; Rameshbabu, N.; Gandhimathi, R. Appl. Water Sci. 2013, 3(1), 1025-1113.
- Benguella, B. H.; Benaissa, H. Water. Res. 2002, 36, 2463–2474.
- Kumar, U.; Bandyopadhyay, M. Bioresour. Technol. 2006, 97, 104-119.
- Zazouli, M. A.;Mahdavi, Y.;Bazrafshan, E. J. Environ. Health. Sci. Eng. 2014,12(66), 1-5.
- Diyanati, R. A.; Cherati, J. Y. J. Mazandaran Univ. Med. Sci. 2013,22(2), 58-64.
- Rozkhash, M.; Eslami, SV. J. Plant Prot. Res.2015, 284,579-588.
- Balarak, D.; Jaafari, J.;Hassani, G.;Mahdavi, Y.;Tyagi, I.; Agarwal, S.; Gupta, V. K. Colloid Interface Sci. Commun.2015,7, 16–19.
- Diyanati, R. A.; Yousefi, Z.; Cherati, J. Y. J. Mazandaran Univ. Med. Sci.2013, 22(2), 13-21.
- Zheng, W.; L i, X. M.; Yang, Q.; Zang, G. M.; Shen, X.; Zhang, Y. J. Hazard. Mater.2007, 147, 534–539.
- Ayuso, E. A.; Sanchez, G. J. Hazard. Mater. 2007, 147, 594–600.
- Diyanati, R. A.; Yousefi, Z.; Cherati, J. Y.; Balarak, D. J. Mazandaran Univ. Med. Sci. 2013, 23, 17-23.
- Azarpira, H.; Mahdavi, Y.; Khaleghi, O. Pharm. Lett.2016, 8(11), 107-113.
- Fuhrman, H. G.; Mikkelsen, P. S.; Ledin, A. Water. Res. 2007, 41, 591–602.
- Balarak, D, Mostafapour, F. K.; Joghataei, A. Pharma. Chem. 2016, 8(8), 138-145.
- Costodes, V. C. T.; Fauduet, H.; Porte, C.; Delacroix, A. J. Hazard. Mater. 2003, 103, 121–142.
- Ashrafi, S. D.; Rezaei, S.; Forootanfar, H.; Mahvi, A. H.; Faramarzi, M. A. IntBiodeter.Biodegr. 2013, 85,173-181.
- Pandey, K.; Sharma, S. K.; Sambi, S. S. Int. J. Environ. Sci. Tech., 2010, 7(2), 395-404.
- Christoforidis, A. K.; Mitropoulos, A.C. Chem. Eng. J. 2015, 277, 334 – 340.
- Mata, Y. N.; Munoz, J. A. Hazard. Mater. 2009, 166, 612 – 618.
- Luo, D.; Xie, Y. F.; Tan, Z. L.; Li, X. D. Environ. Biol. 2012, 34, 359 – 365.
- Balarak, D.; Azarpira, H.; Mostafapour, F. K. Chem. 2016; 8(10):114-121.
- Li, Y. H.; Di, Z. C.; Ding, J.; Wu, D. H.; Luan, Z. K.; Zhu, Y. Q. Res. 2005, 39, 605–609.
- Unlu, N.; Ersoz, M. Hazard. Mater. 2006, 136, 272–280.
- Martins, B. L.; Cruz, C. C. V.; Luna, A. S.; Henriques, A. C. Eng. J. 2006, 27, 310-314.
- Balarak, D.; Mahdavi, Y.; Bazrafshan, E.; Mahvi, A. H. Environ. Bull. 2016, 25(5), 1321-30.
- Meng, Y. T.; Zheng, Y. M.; Zhang, L. M.; He, J. Z. Pollut. 2009, 157, 2577–2583.
- Pacheco, S.; Tapia, J.; Medina, M. R.; Rodriguez, R. Non-Cryst. Solids. 2006, 352, 5475–5481.
- Inbaraj, B. S.; Sulochana, N. Ind J Chem Technol., 2006, 13, 17-23.
- Uddin, S. M.; Azad, A. K.; Firdaus R. Orient J Chem. 2013, 29(2), 241-250.
- Malviya, A.; Kaur, D. Orient J Chem.2012,28(2), 48-57.
- Sirait, M.;Gea, S.; Bukit, N.;Siregar, N.;Sitorus, C. Orient J Chem.2018,34(4), 185-191.

This work is licensed under a Creative Commons Attribution 4.0 International License.









