Solvent Free Green Protocol for the Synthesis of Anti-Bacterial Schiff Base Dimers
1Department of Science and Humanities, Sri Krishna College of Engineering and Technology, Coimbatore, Tamil Nadu, India
2PSG Institute of Technology and Applied Research, Coimbatore, Tamil Nadu, India
Corresponding Author E-mail: manojm@psgitech.ac.in
DOI : http://dx.doi.org/10.13005/ojc/350519
Article Received on : 25-08-2019
Article Accepted on : 29-09-2019
Article Published : 15 Oct 2019
A novel series of Schiff base dimers have been designed and synthesized from phthalaldehydes and various amines including aliphatic, aromatic and heterocycles by grinding at room temperature. This solvent free green protocol approach is superior to the classical approach which uses ethanol as solvent. The advantage of this new method is that wide substrates can be incorporated, very simple to carry out the reaction, easy workup with high product yield. Compounds obtained have been screened for anti-bacterial activity against nine different strains (six Gram-negative and three Gram-positive) which include E.coli, Proteus mirabilis, Klebsiella pneumonia, Proteus vulgaris, Morganella morgana, Salmonella typhi, Staphylococcus aureus, Methicillin-resistant Staphylococcus aureus, Enterococci faecalis. Among them, the heterocyclic groups in the side chain of the Schiff base showed better activity than the aromatic and aliphatic groups. Particularly the thiophene substituted 1,4-isomer of the Schiff base dimer showed good antibacterial activity against seven strains. Interestingly, the 1,3 isomer of the Schiff base dimer with thiophene substituent exhibited a potent antibacterial activity. An effective Structure-Activity Relationship have also been established for the Schiff base dimers to find novel and potent anti-bacterial agents.
KEYWORDS:Anti-Bacterial Activity; Dimers; Schiff Base; Structure-Activity Relationship
Download this article as:| Copy the following to cite this article: Meenakshisundaram S, Manickam M, Solvent Free Green Protocol for the Synthesis of Anti-Bacterial Schiff Base Dimers. Orient J Chem 2019;35(5). |
| Copy the following to cite this URL: Meenakshisundaram S, Manickam M, Solvent Free Green Protocol for the Synthesis of Anti-Bacterial Schiff Base Dimers. Orient J Chem 2019;35(5). Available from: https://bit.ly/2PvQuWF |
Introduction
The chemistry to design dimer molecules for the discovery and development of new drugs or leads for the drugs is proved to be a straightforward and efficient strategy. There are numerous proteins which either exist as hetero/homo dimers or become dimers and perform important functions such as cell proliferation and differentiation. For example, upon activation and binding to the ligand, the receptor tyrosine kinases VEGF and PDGF becomes homodimer and function as important cellular growth factors1,2. Similarly, erythropoietin, a harmone which produce red blood cells binds to two receptors, thus creating a complex with receptor for its function3,4. In the same fashion, α and β isoforms of the estrogen receptor form homo/hetero dimers when they bind to the ligand5-7. The molecular chaperone, Hsp90 exist in the cell as homodimer and responsible for maturation of numerous signaling proteins8. The above examples insist that the therapeutic targets are mostly the dimeric proteins and therefore designing dimer molecules could be a rational strategy to interact/disrupt the protein nature.
There are natural and synthetic dimers that are more active as anti-cancer9, anti-HIV 10, as well as opioidantagonists 11,12 than the monomers. For examples, Indol-3-carbinol13 is commonly found in many vegetables and during digestion it is converted to its dimer diindolylmethane, which then act as an anti-cancer agent14,15. Further exploration of the dimer resulted in a novel anti-cancer agent16 (Fig.1).
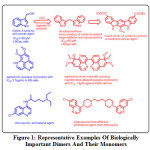 |
Figure 1: Representative Examples Of Biologically Important Dimers And Their Monomers. Click here to view figure |
Aaptamine is an anti-cancer alkaloid isolated from marine sponge Aaptosaaptos 17 while bis-aaptamine, isolated from the marine sponge Aaptossuberitoides showed enhanced cytotoxicity against P388 cell lines (IC50 value = 1.8µM) 18. Chloroquine is being replaced by its dimer, piperaquine for the treatment of malarial disease caused by Plasmodium falciparum 19.
Schiff’s bases are the compounds prepared form amines and aldehydes. They display anti-fungal20, anti-bacterial 21, anti-inflammatory22, anti-viral23 activities and utilized for the synthesis of various heterocyclic compounds such as imidazoles24, imidazopyridines25 and lactams26.
Enviironmentally clean and safe chemical processes are the major goals of the chemists in the prevention of worldwide pollution27. In this regard the most sustainable method is to utilize the principles of Green Chemistry, which is mainly focused on the reduction or elimination chemical by products 28,29. The stable and rapid development of Green Chemistry is based on the environmentally safe and clean products for a long term economy of that country. Particularly pharmaceutically important Schiff bases have been prepared using water as solvent 30.
In view of these above biological importance, an attempt has been made in the present work to synthesize the dimeric analogs of the Schiff bases via a green chemistry approach. All the prepared dimers of Schiff bases, were checked for antibacterial activity.
Results and Discussion
In the first part of the scheme 1, a series of 1,4-isomers of the Schiff bases (5,6) of terephthalaldehyde and aliphatic amines were prepared. In the second part of the scheme 1, a series of 1,3-isomers of Schiff bases (7,8) of isophthalaldehyde and aliphatic amines were prepared. Initially the reaction was carried out in the classical route in which the aldehydes and amines were refluxed in ethanol for 3 hours. The reaction mass was poured into water to get the product as precipitate which was filtered and dried. The yields of the products were only restricted to 70-75%.
In an alternate way for a green chemistry approach, the aldehydes and amines were grinded in a mortar at ambient temperature just for 10 minutes to get the product as precipitate. This solvent free green protocol approach is superior to the classical approach. Simple reaction conditions, easy, clean and safe work up and quantitative yields are the advantages of this new method. The yields were improved to 90-95%. Further to explore the effect of 1,2-positional isomers of the corresponding Schiff bases, we intended to react various amines with phthalaldehyde. However, the reaction did not yield the desired product, probably due to steric factor.
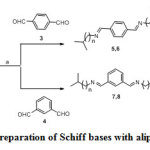 |
Scheme 1: Preparation of Schiff bases with aliphatic amine Click here to view scheme |
Reagents and conditions: a) Classical approach; Ethanol, reflux, 3 h. Green approach; Grinding in mortar for 10 mins.
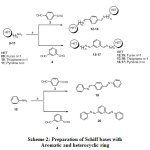 |
Scheme 2: Preparation of Schiff bases with aromatic and heterocyclic ring Click here to view scheme |
Reagents and conditions: a) Classical approach; Ethanol, reflux, 3 h. Green approach; Grinding in mortar for 10 mins.
Table 1: Anti-Bacterial Activity (Zone Of Inhibition In Millimeter) Of The Synthesized Compounds Against Nine Different Strains
|
Compound |
Gram-Negative | Gram-Positive | |||||||
| E.coli | Proteus mirabilis | Klebsiella pneumonia | Proteus vulgaris | Morganella morgana | Salmonella typhi | Staphylococcus aureus | Methicillin-resistant Staphylococcus aureus |
Enterococci faecalis |
|
|
5 |
0 | 0 | 0 | 0 | 0 | 0 | 18 | 17 | 0 |
| 6 | 0 | 10 | 0 | 14 | 0 | 0 | 16 | 15 |
0 |
|
7 |
0 | 10 | 0 | 0 | 13 | 0 | 10 | 12 | 0 |
| 8 | 0 | 15 | 0 | 18 | 12 | 0 | 16 | 18 |
16 |
|
12 |
0 | 10 | 18 | 16 | 12 | 0 | 0 | 12 | 0 |
| 13 | 10 | 28 | 24 | 28 | 26 | 0 | 25 | 28 |
25 |
|
14 |
10 | 22 | 18 | 22 | 18 | 0 | 22 | 20 | 15 |
| 15 | 0 | 0 | 0 | 0 | 0 | 0 | 18 | 17 |
0 |
|
16 |
10 | 28 | 26 | 28 | 26 | 0 | 26 | 26 | 20 |
| 17 | 0 | 10 | 0 | 0 | 13 | 0 | 10 | 12 |
0 |
|
19 |
0 | 13 | 0 | 17 | 13 | 0 | 15 | 18 | 15 |
| 20 | 0 | 16 | 12 | 20 | 12 | 0 | 18 | 20 |
18 |
|
Amoxicillin |
30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Ceftriaxone | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
30 |
The anti-microbial activity for the prepared compounds was examined against nine different strains (six Gram-negative and three Gram-positive, Table 1). Among them, aliphatic derivatives of 1,4-positional Schiff base isomers (5,6) and 1,3-positional Schiff base isomers (7,8) did not show good activity. The introduction of heterocyclic rings on both positions like furan, thiophene and pyridine showed better activity when compared to the phenyl rings itself. Among them the thiophene ring moiety 13,16 showed excellent activity. When compared to the 1,4-Schiff base isomer of thiophene (13) the 1,3-Schiff base isomer (16) exhibited the best activity along all strains except for Salmonella typhi. Figure 2 represents the zone of inhibition of the potent compound 13 against 8 different bacteria.
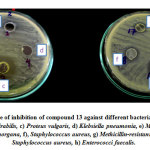 |
Figure 2: Zone of inhibition of compound 13 against different bacteria Click here to view figure |
Conclusion
A solvent free green protocol has been designed to access the Schiff base dimers. In comparison of the classical approach in which refluxing ethanol has been used as reaction condition to get the yield of 70-75%, the green synthesis utilize grinding the reactants at room temperature with the output of high yield 90-95%. Simple reaction conditions, substrate generality, easy, safe and clean work up procedure with quantitative yields are the advantages of this novel method. With this method, a series of Schiff base dimers have been prepared. The aliphatic substituted compounds 5-8 did not exhibit good activity. However the heteroaromatic substituted Schiff bases 12-17 showed moderate to good activity. When compare to simple aromatic phenyl ring dimers 19,20 the heteroaromatic Schiff base dimers showed potential activity. Among them, the thiophene substituted 1,4-isomer of the Schiff base 13 showed good antibacterial activity for seven strains. The 1,3 isomer 16 exhibited a potent antibacterial activity. Thus a green protocol has been designed to synthesize highly potent antibacterial Schiff base dimers.
Acknowledgement
The authors thank IISc, Bangalore, Hyderabad Central University for NMR analysis.
Conflicts of Interest
No potential conflict of interest was reported by the authors.
References
- Schlessinger, J. Cell. 2000,103, 211-225.
- Wiesmann, C.; Fuh, G.; Christinger, H.W.; Eigenbrot, C.; Wells, J.A.; de Vos, A.M. Cell. 1997, 91, 695-704.
- Kossiakoff, A.A.; de Vos, A.M. Adv. Protein Chem. 1998, 52, 67-94.
- Jiang, G.; Hunter, T. Curr. Biol. 1999, 9, R568-R571.
- Hall, J.M.; Couse, J.F.; Korach, K.S. J. Biol. Chem. 2001, 276, 36869-36872.
- Tamrazi, A.; Carlson, K.E.; Daniels, J.R.; Hurth, K.M.; Katzenellenbogen, J.A. Mol. Endocrinol. 2002, 16, 2706-2719.
- Brandt, M.E.; Vickery, L.E. J. Biol. Chem. 1997, 272, 4843-4849.
- Blagg, B.S.; Kerr, T.D. Med. Res. Rev. 2006, 26, 310-338.
- Gamage, S.A.; Spicer, J.A.; Atwell, G.J.; Finlay, G.J.; Baguley, B.C.; Denny, W.A. J. Med. Chem., 1999, 42, 2383-2393.
- Xu, J-B.; Zhang, H.; Gan, L.S.; Han, Y.S.; Wainberg, M.A.; Yue, J-M. J. Am. Chem. Soc. 2014, 136, 7631-7633.
- Neumeyer, J. L.; Zhang, A.; Xiong, W.; Gu, X-H.; Hilbert, J.E.; Knapp, B.I.; Negus, S.S.; Mello, N.K.; Bidlack, J.M. J. Med. Chem. 2003, 46, 5162-5170.
- Berube, G. Curr. Med. Chem. 2006, 13, 131-154.
- Hung, W.C.; Chang, H.C. J.Agric. Food Chem. 2009, 57, 76–82.
- Grose, K.R.; Bjeldanes, L.F. Chem. Res. Toxicol. 1992, 5, 188–193.
- Stuab, R.E.; Onisko, B.; Bjeldanes, L.F. Chem. Res. Toxicol. 2006, 19, 436–442.
- Chao, W.R.; Yean, D.; Amin, K.; Green, C. J. Med. Chem. 2007, 50, 3412–3415.
- Shaari, K.; Ling, K.C.; Rashid, Z.M.; Jean, T.P.; Abas, F.; Raof, S.M.; Zainal, Z.; Lajis, N.H.; Mohamad, H.; Ali, A.M. Mar. Drugs. 2009, 7, 1-8.
- Liu, C.; Tang, X.; Li, P.; Li, G. Org. Lett. 2012, 14,1994-1997.
- Basco, L.K.; Ringwald, P. Antimicrob. Agents Chemother. 2003, 47, 1391–1394.
- Karthikeyan, M.S.; Prasad, D.J.; Poojary, B.; Bhat, K.S.; Hollaa, B.S.; Kumari, N.S. Bioorg. Med. Chem. 2006, 14, 7482–7489.
- Vinita, G.; Sanchita, S.; Gupta, Y.K.; Res. J. Chem. Sci. 2013, 3, 26-29.
- Sahoo, B.M.; Dinda, S.C.; Ravi Kumar, BVV.; Panda, J.; Brahmkshatriya, P.S.; Lett. Drug Des. Discov. 2014, 11, 82-89.
- Suresh Kumar, K.; Ganguly, S.; Veerasamy, R.; Clercq, E.D. Eur. J. Med. Chem. 2010, 45, 5474-5479.
- Almansa, C.; Alfon, J.; de Arriba, A.F.; Cavalcanti, F.L.; Escamilla, I.; Gomez, L.A.; Miralles, A.; Soliva, R.; Bartrolı, J.; Carceller, E.; Merlos, M.; Rafanell, J.G. J. Med. Chem. 2003, 46, 3463-3475.
- Guchhait, S.K.; Chandgude, A.L.; Priyadarshani, G. J. Org. Chem. 2012, 77, 4438−4444.
- Naser A.W.; Majeed, A.M. J. Chem. Pharm.Res. 2015, 7, 300-306.
- Rio Declaration on Environment and Development, Rio de Janeiro, Brazil, June 3-14, 1992 (http://www.unesco.org/education/information/nfsunesco/pdf/RIO_E.PDF).
- Anastas, P.T.; Warner, J.C.; GreenChemistry,Theory and Practice, Oxford University Press, Oxford, 1998.
- Horváth, I.T. Acc.Chem.Res. 2002, 35, 685.
- Cue, B.W.; Zhang, J. Green Chem. Lett. Rev. 2009, 2, 193-211.

This work is licensed under a Creative Commons Attribution 4.0 International License.










