Cerium Oxide Nanoparticles Catalyst for the Oxidation of Methanol
Chemistry Department, Faculty of Science, Albaha University, Albaha, Kingdom of Saudi Arabia.
Corresponding Author E-mail: hbayahia@bu.edu.sa
DOI : http://dx.doi.org/10.13005/ojc/350510
Article Received on : 04-07-2019
Article Accepted on : 02-09-2019
Article Published : 04 Sep 2019
This study outlines the synthesis of cerium oxide nanoparticles, their characterization and their activity in the oxidation of methanol. A simple and easy co-precipitation method was used for the preparation of cerium oxide, without any added surfactants. The physicochemical properties of the sample were studied using scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDX) and X-ray diffraction (XRD). The morphology and size of the catalyst was studied using SEM. EDX confirms the element content of the synthesized cerium oxide. The structure of CeO2 was confirmed using XRD. Thus, the reported CeO2 was an active catalyst for methanol oxidation to form formaldehyde at a temperature range of 523–753K in the gas phase. At 753K, the cerium oxide catalyst gave 53% formaldehyde selectivity, 57% methanol conversion and 31% formaldehyde yields.
KEYWORDS:Catalyst; Cerium Oxide; Formaldehyde; Oxidation Of Methanol; Nanoparticles
Download this article as:| Copy the following to cite this article: Bayahia H. Cerium Oxide Nanoparticles Catalyst for the Oxidation of Methanol. Orient J Chem 2019;35(5). |
| Copy the following to cite this URL: Bayahia H. Cerium Oxide Nanoparticles Catalyst for the Oxidation of Methanol. Orient J Chem 2019;35(5). Available from: https://bit.ly/2JtdVfF |
Introduction
Different rare-earth oxides have different nanostructures and different applications and studies report that their chemical characterization arises from their 4f electrons [1-5]. Compared with the other nanostructured lanthanide oxides, Cerium Oxide nanocrystals have been widely investigated as important functional materials [6, 7].
The nanomaterials of cerium oxide have attracted much interest due to their wide-ranging applications [8-15]. Cerium oxide has a wide range of applications, such as decolonizing glasses, infra-red filters, energy storage and the electrical, electronic and battery industries. It is also used as a catalyst and for adsorption in many chemical reactions, and has different and useful promising applications in many biomedical fields [16-19].
Various methods – such as hydrothermal synthesis, spray pyrolysis, surfactant-templated synthesis, solid-state reactions, mechanochemical reactions, sonochemical synthesis, microwave irradiation, ball milling, combustion synthesis – have been suggested for the synthesis of diverse CeO2-based nanomaterials [20-24].
The oxidation of methanol to formaldehyde is significant: formaldehyde is an important product because of its industrial applications and its role in producing other useful chemicals such as urea-phenolic acetal. The amount of formaldehyde produced worldwide by oxidation of methanol is around 4–5 107 tons per year. Different types of catalysts have been used and tested in the oxidation of methanol to produce formaldehyde and other products, such as hydrogen and carbon dioxides [25]. The effectiveness of the alcohol selective oxidation reaction in aqueous hydrogen peroxide has been investigated using Rh-bis (benzidazole) prydinedicarboxlate complex as the catalyst, using different solvents, temperatures and quantities of catalyst. Polar solvents, such as acetonitrile and ethanol, gave better yields of corresponding carbonyl compounds compared to less polar solvents, such as toluene and ethyl acetate. However, solvent-free system gave the best product yields: for example, the benzaldehyde yield was 96% with the solvent-free method, compared to 74% using acetonitrile solvent. The mole ratio of alcohols to H2O2, as well as the temperature and quantity of catalyst, affect this reaction. Increasing the reaction temperature and amount of catalyst led to increased product yields [26]. Using a thiophene-based covalent triazine framework as free toxic metals in the selective oxidation of benzyl alcohols under visible light in the presence of oxygen and at room temperature gave 100% benzaldehydes yields [27].
V2O5-MoO3 with different V/Mo ratios and its supported catalysts were tested for producing of formaldehyde by oxidation of methanol at low temperature. In this work, both V and Mo played a role in the reaction: MoO3 enhanced Brɵnested acid sites on the surface of catalysts and V enhanced the oxygen capacity in the reaction. This process gave good oxidation of methanol reaction selectivity, which was 92% at low reaction temperature (120 °C), and the catalysts were active in the reaction [28]. The oxidation of methanol was carried out with Ag (110) and Cu (110) catalysts at 250–340K to produce formaldehyde. The results showed that at constant rate, Ag had a higher surface, but Cu showed stronger catalytic absorbate as it has a different surface adsorption of oxygen and methanol [29]. Supported Ag-nanoparticle catalysts have been tested and reported in the oxidation of methanol to form formaldehyde using different Ag loadings at higher temperatures of 560–650 °C. The results showed that, at 650 °C, the yield of formaldehyde was increased to around 85% [30]. Ferric tungstate and ferric molybdate have been tested in the oxidation of methanol under the same conditions. It was found that ferric molybdate produced mainly formaldehyde, while ferric tungstate produced dimethyl ether [31]. This catalyst was selective and both phases of MoO3 and Fe2O3 are important: MoO3 for selective formaldehyde and Fe2O3 for methanol conversion [32].
Recently, aldehydes, ketones and carboxylic acids were produced by oxidation of alcohols using hydroxyl ammonium as a catalyst, in conjunction with sodium hypochlorite-5-haydates as the terminal oxidant and acetonitrile with water as the solvent. The reaction completed within half an hour to two hours with good yields of products [33]. To improve the oxidation of alcohols, direct oxidation of alcohols with hydrogen was electrochemically tested in the continuous flow reactor without any additives. The results showed a very high yield of corresponding carbonyl compounds. Different currents have been trialed in the reaction, ranging from 10–800 mA. At 800 mA, using carbon cloths and nickel plates, the benzaldehyde yield was 99% for 25-minute reaction times [34]. Electrocatalytic oxidation of C1-C3 and polybasic alcohols was investigated using different electrodes such as Pt, Pd, and Au, and using acidic, natural and alkaline media. It has been found that the best selectivity of formaldehyde by oxidation of methanol took place with Pt, but for alcohols with higher amounts of carbon C2-C3, Pd was the active electrode, while Au was active for oxidation reactions of polybasic alcohols in the alkaline medium [35].
In our work, we synthesized cerium oxide nanomaterials using the simple and easy co-precipitation method, characterized it using scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDX) and X-ray diffraction (XRD) methods, and tested it for the oxidation of methanol to form formaldehyde at range of temperatures. To our knowledge, the oxidation of methanol to form formaldehyde with a cerium oxide catalyst has never been investigated
Materials and methods
Chemicals and materials
Cerium sulfate (Sigma-Aldrich, ≥99.99%), sulfuric acid (PanReac, 95–98%), extra pure ammonia solution (Loba Chemie), and distilled water were used in this work, without any additional purification processes.
Cerium oxide nanoparticle synthesis
For the synthesis of cerium oxide nanoparticles, a co-precipitation method was used. First, 3.32g of cerium sulfate was dissolved in 2M of sulfuric acid. That mixture was diluted to 50 mL with distilled water. The solution was continuously stirred using a magnetic stirrer until the cerium sulfate was completely dissolved and a clear solution was achieved. After the dissolution of the cerium sulfate, ammonia solution (extra pure, 30%) was added dropwise with constant stirring, until the solution pH reached 10–12. The resulting solution was stirred for an aging time of 1 hour and washed four times: three times with distilled water and the fourth time with ethanol, to remove the byproducts and some impurities. After that, the product was dried in an oven at 80 °C overnight. Finally, the sample was calcined at 400 °C for 4 hours to obtain cerium oxide. The following schematic diagram shows the synthesis steps of cerium oxide.
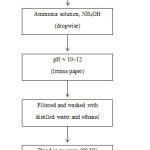 |
Scheme 1 Click here to View scheme |
Schematic diagram for the synthesis of CeO2 nanoparticles
Catalyst testing
The catalytic reaction of methanol oxidation was carried out in a stainless steel reactor located in the center of a furnace, fed from the top using oxygen mixed with nitrogen (O2 and N2 at a ratio of 80% to 20% with a flow rate of 20 mL/min). The molar ratio of O2/methanol was 0.52, using a range of temperature 523–753K for 5 hours’ time on stream (TOS). The products were analyzed using gas chromatography (Varian 3800 with a capillary column and flame ionization detector).
Results and discussion
Catalyst characterization
The structure of the synthesized cerium oxide was studied using XRD [32]. The patterns of synthesized cerium oxide nanoparticles confirmed the structure of CeO2 as shown in Fig. 1. Positions of all peaks in the XRD pattern of cerium oxide matched data for the standard CeO2 crystal structure in JCPDS card No. 81-0792, and they also showed the same results as reported by Z. L. Zhang et al. [36]. No noticeable peaks were detected indicating the formation of single-phase CeO2 with a cubic structure (Fig. 1).
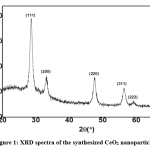 |
Figure 1: XRD spectra of the synthesized CeO2 nanoparticles Click here to view figure |
Fig. 2 shows the surface and morphology of the cerium oxide obtained using SEM and the shape of cerium oxide was confirmed [32, 36]. The cerium oxide particles were confirmed as nanoparticles, as shown in Fig. 2. EDX shows the composition of cerium oxide elements that were prepared and used in this work: Fig. 3 shows high intensified peaks for cerium and oxygen in the EDX spectrum (similar results have been previously reported [37]), and a small peak related to sulfur, which came from the sulfuric acid used for dissolving the cerium sulfate during the catalyst synthesis.
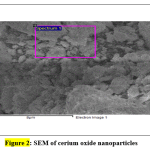 |
Figure 2: SEM of cerium oxide nanoparticles Click here to view figure |
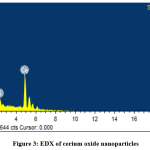 |
Figure 3: EDX of cerium oxide nanoparticles Click here to view figure |
Cerium oxide and oxidation of methanol
In this work, the cerium oxide catalyst was tested in methanol oxidation in the gas phase using 0.5g of catalysts, in the presence of oxygen. The products were analyzed, and the results showed that the catalysts were active at different temperatures in the range of 523–753K for 5 hours’ TOS. It was noted that the conversion of methanol increased when the reaction temperature was raised. The highest conversion of methanol was at the highest temperature applied in this study, which was 753K. Fig. 4 shows that, at 523K, the conversion of methanol over cerium oxide was 25%, and it increased to 57% at 753K.
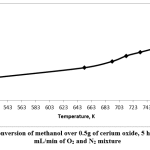 |
Figure 4: Conversion of methanol over 0.5g of cerium oxide, 5 hours’ TOS, 20 mL/min of O2 and N2 mixture Click here to view figure |
Formaldehyde selectivity is shown in Fig. 5. The selectivity decreased with increasing temperature. At 523K, formaldehyde selectivity was 77%. However, it decreased when the temperature was increased from 523K to 753K with 5 hours’ TOS, gradually dropping to 53% at 753K.
Fig. 6 shows the formaldehyde yields. It can be seen that the highest formaldehyde yield was at 753K, which was 31% (conversion of methanol was 57% and selectivity of formaldehyde was 53%).
2.0 wt% of Ag/TiO2 gave 60% methanol conversion at 69% formaldehyde selectivity at 673K, but the catalyst showed higher conversion and selectivity by increasing the load of Ag/TiO2 from 2.0 to 12.0 wt% using the same reaction conditions[25]. These results are similar to the activity of CeO2 nanoparticle catalysts for methanol oxidation to form formaldehyde in this study.
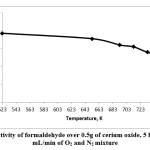 |
Figure 5: Selectivity of formaldehyde over 0.5g of cerium oxide, 5 hours’ TOS, 20 mL/min of O2 and N2 mixtureClick here to view figure |
Green protocol of the selective oxidation of various alcohols in the system of quinaldinium chromate, periodic acid (H5IO6), and solvent-free has been previously investigated. The oxidation of benzyl alcohol gave a 96% benzaldehyde yield in a 0.5-hour reaction time at room temperature [38]. This good product yield result, in comparison to the results in the present study, might be due to only bulk cerium oxide being used in the gas phase, without any supported catalyts.
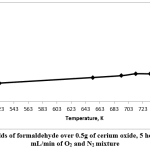 |
Figure 6: Yields of formaldehyde over 0.5g of cerium oxide, 5 hours’ TOS, 20 mL/min of O2 and N2 mixture Click here to view figure |
Electrical oxidation of benzyl alcohol using carbon cloths and nickel plate at 800 mA and 0.05 mL/s flow rate gave a benzaldehyde yield of 67%. The yield of aldehyde was increased to 99% by increasing the flow rate to 0.1 mL/s and the current to 1000 mA [34]. Oxidation of a range of alcohols over a (NH4)5[IMo6O24] catalyst was investigated using acetonitrile and water as a solvent and a 10% NaCl and oxygen balloon at 70 °C for a 12-hour reaction time. The yield of benzaldehyde (as one example of the products) was 96%, but when the additives changed, the products yields also changed, so the additives can be used to control the product yields [39].
Conclusion
In summary, a simple and easy co-precipitation method has been developed for the synthesis of CeO2 nanomaterials using ammonia as the precipitating agent. The cerium oxide nanoparticle catalyst was characterized using techniques such as XRD, SEM and EDX. The catalyst was active in the oxidation of methanol and it gave 53% formaldehyde selectivity at 57% methanol conversion at 753K.
Acknowledgements
I would like to thank the Center of Nanotechnology at King Abdulaziz University, Saudi Arabia for helping with the characterization of cerium oxide and I am also thankful to Dr. Malik Shamshi Hassan at Albaha University for scientific talk regarding CeO2 nanoparticle synthesis.
Conflicts of Interest
The author declares that there are no conflicts of interest in this article.
References
- Wu, G. S.; Zhang, L. D.; Cheng, B. C.; Xie, T.; Yuan, X. Y. Synthesis of Eu2O3 Nanotube Arrays through a Facile Sol−Gel Template Approach., Am. Chem. Soc., 2004, 126, 5976-5977.
- Can, X.; Xianogang Q. P.; Cerium oxide nanoparticles: a markably versatile rare earth nanomaterials for biological applications, NPG Asia. Mater., 2014, 6, 1-16.
- Atul D.; William S.; Cerium Oxide Nanoparticles: A Brief Review of Their Synthesis Methods and Biomedical Applications, Antioxidants, 2018, 7(97), 1-13.
- Yu, M.; Lin, J.; Fu, J.; Zhang, H. J.; Han, Y. C., Sol–gel synthesis and photoluminescent properties of LaPO4: A (A = Eu3+, Ce3+, Tb3+) nanocrystalline thin films, Mater. Chem., 2003, 13, 1413–1419
- Preeti S.; Abdullah M. M.; Saiqa I., Role of Nanomaterials and their Applications as Photo-catalyst and Senors: A Review, Nano Res. & Appl., 2016, 2, 1-10.
- Yu, T.; Joo, J.; Park, Y. I.; Hyeon, T., Large‐Scale Nonhydrolytic Sol–Gel Synthesis of Uniform‐Sized Ceria Nanocrystals with Spherical, Wire, and Tadpole Shapes, Chem., 2005, 117, 7577 –7580.
- Wang, Z. L.; Feng, X. D. J., Polyhedral shapes of CeO2 nanoparticle, Chem. B., 2003, 107, 13563-13566.
- Mamontov, E.; Egami, T.; Brezny, R.; Koranne, M. Tyagi, S., Lattice Defects and Oxygen Storage Capacity of Nanocrystalline Ceria and Ceria-Zirconia, Phys. Chem. B., 2000, 104, 11110-11116.
- Tsunekawa, S.; Fukuda, T.; Kasuya, A., Blue shift in ultraviolet absorption spectra of monodisperse CeO2−x nanoparticles, J. Appl. Phys., 2000, 87, 1318–1321.
- Hsu, W. P.; Ronnquist, L.; Matijevic´, , Preparation and properties of monodispersed colloidal particles of lanthanide compounds Cerium(IV), Langmuir, 1988, 4, 31-37.
- Masui, T.; Fujiwara, K.; Machida, K.; Adachi, G.; Sakata, T.; Mori,H. Characterization of Cerium(IV) Oxide Ultrafine Particles Prepared Using Reversed Micelles, Mater., 1997, 9, 2197-2204.
- Vimal K. P.; Mani, P. R.; Biju, C. J.; Unnikrishnan V.; Ittyachen, M. A., Structural studies and luminescence properties of CeO2:Eu3+ nanophosphors synthesized by oxalate precursor method, Appl. Nanosci., 2015, 5, 837–846
- Li, F.; Yu, X. H.; Pan, H. J.; Wang, M. L.; Xin, X. Q., Syntheses of MO2 (M=Si, Ce, Sn) nanoparticles by solid-state reactions at ambient temperature, Solid State Sci., 2000, 2, 767-772.
- Li, Y. X.; Zhou, X. Z.; Wang, Y.; You, X. Z., preparation of nanosize CeO2 by mechanochemical reaction of cerium carbonate with sodium hydroxide, Lett., 2003, 58, 245-249.
- Farahmandjou, M.; Zarinkamar, M.; Firoozabadi, T. B., Synthesis of Cerium Oxide (CeO2) nanoparticles using simple CO-precipitation method, Mex. Fis., 2016, 62, 496–499.
- Xiutong, F.; Hongxing, S., Synthesis of cerium oxide nanoparticles loaded on chitosan for enhanced auto-catalytic regenerative ability and biocompatibility for the spinal cord injury repair, Photochem. Photobiol. B, Biol., 2019, 191, 83-87.
- Rajeshkumar, S.; Poonam, N.; Synthesis and biomedical applications of Cerium oxide nanoparticles – A Review, Biotechnol. Rep. (Amst)., 2018, 17, 1–5.
- Jessica T. D.;Yuji A. Environmental Geochemistry of Cerium: Applications and Toxicology of Cerium Oxide Nanoparticles, Int. J. Environ. Res. Public Health, 2015, 12, 1253-1278.
- Xia, B.; Lenggoro, I. W.; Okuyama, K., Synthesis of CeO2 nanoparticles by salt-assisted ultrasonic aerosol decomposition, Mater. Chem., 2001, 11 (12), 2925–2927.
- Hirano, M.; Kato, E., Hydrothermal Synthesis of Nanocrystalline Cerium(IV) Oxide Powders, Am. Ceram. Soc., 1996, 79, 777–780.
- Gesser, H. D.; Goswami, P. C., Aerogels and related porous materials, Rev., 1989, 89, 765–788.
- Li, Gao-Ren; Qu, Dun-Lin; Arurault, L.; Tong, YeX.; Hierarchically Porous Gd3+-Doped CeO2 Nanostructures for the Remarkable Enhancement of Optical and Magnetic Properties, Physi. Chem. C., 2009, 113, 1235-1241.
- Yadav, T.; Srivastava, O., Synthesis of nanocrystalline cerium oxide by high energy ball milling. Int., 2012, 38, 5783–5789.
- Wang, Y.; Mori, T.; Li, J.G.; Ikegami, T., Low-temperature synthesis of praseodymium-doped ceria nanopowders. Am. Ceram. Soc. 2002, 85, 3105–3107.
- Maldonado, C, Fierro, J. G.; Birke, G; Martinez, E.; Reyes, P., Conversion of methanol to formaldehyde on TiO2 supported Ag nanoparticles, Chil. Chem. Soc., 2010. 55, 506-510.
- Zhou, X. T.; Ji, H-B.; Liu, Sh-Gu. Solvent-free selective oxidation of primary and secondary alcohols catalyzed by ruthenium-bis(benzimidazole)prydidnecarboxylate complex using hydrogen peroxide as an oxidant, Tetrahedron Lett., 2013, 54, 3882-3885.
- Huang, W.; Ma, B. C.; Lu, H.; Li, R.; Wang, L.; Landfester, K.; Zhang, K. A. I. Visible-Light-Promoted Selective Oxidation of Alcohols Using a Covalent Triazine Framework, ACS Catal., 2017, 7, 5438−5442.
- Yali M. T.; Wang S. Chen Y.; Zhao X. M., Selective Oxidation of Methanol to Dimethoxymethane on V2O5-MoO3/γ-Al2O3 Catalysts, Catal. B: Environ., 2014, DOI: http://dx.doi.org/doi:10.1016/j.apcatb.2014.05.008
- Wachs, i. E.; Robert J. M., The oxidation of methanol on a silver (110) catalyst, Sci. 1978, 76, 531-558.
- Tom W.; Richard A.; J. O.; Anthony G. W., Catalytic Gas Phase Oxidation of Methanol to Formaldehyde, Am. Chem. Soc., 2003, 125 , 3384-3396.
- Catherine, B.; Michael, B.; Peter P. W., Catalysts for the Selective Oxidation of Methanol, Catal., 2016, 6, 1-27.
- Cheliah, M; Rayappan, B. B.; Krishnan, U. M., Synthesis and cauterization of Cerium Oxide Nanoparticles by Hydroxide Mediated Approach, J. Appl. Sci., 2012, 12 (16), 1734-1737.
- Miller, S.; Bisset, K.; Leadbeater, N.; Eddy, N. Catalytic Oxidation of Alcohols Using a 2,2,6,6Tetramethylpiperidine-N-hydroxyammonium Cation, Eur. J. Org. Chem., 2019, 10.1002/ejoc.201801718.
- Wang, D.; Wang, P.; Wang, S.; Chen, Y.; Zhang, H.; Lei, A. Direct electrochemical oxidation of alcohols with hydrogen evolution in continuous-flow reactor, Commun., 2019, 10, 1- 8.
- Wang, B.; Tao, L.; Cheng, Y.; Yang, F.; Jin, Y.; Zhou, C.; Yu, H.; Yang, Y. Electrocatalytic Oxidation of Small Molecule Alcohols over Pt, Pd, and Au Catalysts: The Effect of Alcohol’s Hydrogen Bond Donation Ability and Molecular Structure Properties, Catal., 2019, 9(387), 1-18.
- Zhang, J.; Ju. X.; Wu. Z., Y.; Liu, T.; Hu, T. D.; Xie, Y. N.;. Zhang, Z. L., Structural Characteristics of Cerium Oxide Nanocrystals Prepared by the Microemulsion Method, Mater., 2001, 13, 4192-4197.
- Sangsefidi, F. S; Nejati, M; Verdi, j.; Niasari, M. S. Clean. Produ., 2017, 156, 741-749.
- Canbulat Özdemir M. Quinaldinium Chlorochromate(VI), (QnCC) Catalyzed Oxidation of Alcohols with Periodic Acid Under Solvent-Free Conditions and Microwave Irradiation, Turkish Chem., 2018, 5(2):, 679-690.
- Zhang, M.; Zhai, Y.; Ru, S.; Zang, D.; Han, S.; Yu, H.; Wei, Y. Highly practical and efficient preparation of aldehydes and ketones from aerobic oxidation of alcohols with an inorganic-ligand supported iodine catalyst, Chem. Commun., 2018, 54, 10164-10167.

This work is licensed under a Creative Commons Attribution 4.0 International License.










