Catalytically Active Gold Nanoparticles Synthesized using Diabetic Sugar Via Rapid, Simple, Greener Process
1Department of Industrial Waste Treatment Polytechnic of AKA Bogor, Bogor 16154, Indonesia
2Chemical Analysis Department, Polytechnic of AKA Bogor, Bogor 16154, Indonesia
Corresponding Author E-mail: nurdiani97@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350507
Article Received on : 29-08-2019
Article Accepted on : 30-09-2019
Article Published : 14 Oct 2019
Au nanoparticles were successfully synthesized using diabetic sugar as reducing agent, with and without the aid of microwave heating. The hydrolysis of diabetic sugar was carried out using HCl, prior to the synthesis. By adjusting pH of the mixture to basic condition, the formation of the nanoparticles effectively occurred resulting purple colloidal solution. The solution was centrifuged or left overnight to yield precipitate of Au nanoparticles. The as-formed Au nanoparticles were characterized by UV-Vis spectrophotometry, Fourier-Transform infrared spectrophotometry and scanning electron microscopy. The result showed that the Au nanoparticles played a significant role as catalyst for oxidation of methylene blue by H2O2. The kinetics of the oxidation process followed pseudo first order reaction rate with the rate constant depending on the AuNP characteristic and experimental condition.
KEYWORDS:Au Nanoparticles; Catalyst; Diabetic Sugar; Green Chemistry; Methylene Blue;Synthesis
Download this article as:| Copy the following to cite this article: Nurdiani N, Foliatini F. Catalytically Active Gold Nanoparticles Synthesized Using Diabetic Sugar Via Rapid, Simple, Greener Process. Orient J Chem 2019;35(5). |
| Copy the following to cite this URL: Nurdiani N, Foliatini F. Catalytically Active Gold Nanoparticles Synthesized Using Diabetic Sugar Via Rapid, Simple, Greener Process. Orient J Chem 2019;35(5). Available from: https://bit.ly/2Nsv9La |
Introduction
Several studies have been carried out to explore the highly potential Au nanoparticles as catalyst1. Au nanoparticles are often combined with other materials to improve stability and performance as a catalyst. Previous studies reported Au nanoparticle catalysts in both nanocomposites and bimetallic forms, such as Au/dendrimer2 , Au/thiol3 , Rh/Au4 , and Au/Cu5. Several factors have been shown to have a significant effect on the activity of Au nanoparticles as catalysts, among others, nanoparticle size and supporting material characteristics6. Previous research explained that Au nanoparticles have a high catalytic activity against several chemical reactions if they have a size of less than 3-5 nm7. Research carried out by7 shows that on the Au nanoparticles particle size 2-5 nm the catalysis efficiency is very high and decreases dramatically at a larger size. Other studies have shown that particle morphology such as shape, uniformity in size and shape, and the existence of certain active sides can play a considerable role in efficiency6.
Synthesis of Au nanoparticles in the absence of synthetic chemicals are preferable since it reduces chemical waste, more biodegradable, cost-effective and non-toxic. The main advantage of the green synthesis is the absent of harmful chemicals thus do not make a negative impact to the environment. For this reason, in recent years, biosynthesis of nanoparticles has received considerable attention. Under the experimental condition, spherical nanoparticles of approximately 1, 3, 10 and 20 nm sizes were prepared reproducibly. Several monosaccharide (ribose, fructose, sorbose, glucose, xylose, and galactose) were also reported to succesfully reduces Ag+ to produce antibacterial silver nanoparticles with particle size of 10 to 35 nm9. Commercial sugar produced from plants, such as sugarcane and sugar beets, has also been used as reducing agent in the nanoparticle synthesis, 10 have developed the greener method in the synthesis Ag nanoparticles using white sugar, such kind of refined sugar.
In this research, we reported the general method for the synthesis of Au nanoparticles using diabetic sugar as reducing agent in alkaline condition, with and without the aid of microwave heating process. The as-obtained Au nanoparticles acted as catalyst in the oxidation of methylene blue by H2O2.
Experimenital
Materials and Methods
Materials
Solid pure gold (99,99%) was obtained from PT. Antam Tbk, Commercially diabetic sugar was bought from local market in Bogor, Indonesia, HNO3, HCl, NaOH, methylene blue and hydrogen peroxide were purchased from Merck. All chemicals used in this experiment were of analytical grade. Double distilled water was used as solvent for preparation of sugar and metal precursor solution.
UV-Visible spectrophotometer (UV-1700, Shimadzu) was used to characterize the Au nanoparticles and monitor the kinetics of methylene blue oxidation. The characterization of functional groups and interaction between Au nanoparticles and sugar were determined by Fourier-transform infra red (Bruker).
Methods
Synthesis of Au Nanoparticles
Synthesis of Au nanoparticles is carried out with and without the aid of microwaves at certain irradiation power. A total of 5 mL of 1 mM HAuCl4 solution was put into a beaker glass then added by 30 mL of 15% sugar. The pH of the mixture adjusted with NaOH 0.1 M until alkaline condition reached. The beaker glass was put into a microwave oven then warmed up for 2-3 minutes. Changing the color from clear yellow to red or purple showed that Au nanoparticles have been formed. The formed Au nanoparticle colloids were centrifuged at 3000 rpm for 30 minutes and ready to be characterized. For assaying the catalytic activity, the colloidal Au nanoparticle solution was kept overnight and the precipitated was sieved and dried.
Oxidation of methylene blue in the presence of Au nanoparticles catalyst
As much as 0.05 grams of Au nanoparticles mixed thoroughly with 20 mL of 3 ppm methylene blue solution and stirred for several minutes. After that, 1 mL of 30% hydrogen peroxide solution was added to the mixture and the stirring was done again. The oxidation process of methylene blue was monitored by measuring its absorbance at a wavelength of 664 nm. The absorbance measurement is carried out every 10 minutes for 1-3 hours. Data retrieval is done twice for oxidation with and without catalyst.
Results and Discussion
Synthesis of Au Nanoparticles
Several studies have been conducted to propose general scheme for mono- and disaccharide aided- metal nanoparticles synthesis. The usage of commercial sugar for the same goal, in which the complex matrix may involve in the reaction process, was rarely studied. The research aimed to explore the effect of sugar matrix in the commercial sugar sample on the effectivity of the Au nanoparticles synthesis. Commercial sugar such as white sugar was produced from plants, and further processed to remove various impurities. In the nanoparticle synthesis steps, sucrose from the sugar will be hydrolyzed in alkaline condition, resulting two reducing sugar, fructose and glucose, which played a role to reduce cation of metal precursors10.
In this experiment, the artificial sweetener for diabetic patients, sucralose, such a chlorinated carbohydrate, was utilized as reducing agent, instead of white sugar. Since the substance must be hydrolyzed to become useful reducing agent in the nanoparticles synthesis, the NaOH was added to adjust the alkaline condition as required for hydrolysis. The previous literature reported the stability of the sucralose in the pH of 3-711. Thus, in the experiment we focused on the synthesis at high pH, though the low pH was also studied to prove the above statement.
Most of the green protocols reported using sugars or starch for the synthesis of metal nanoparticles involve the use of direct heat treatment (conventional heating) or autoclave, taking relatively few minutes to hours for the synthesis. Therefore exploitation of such a method of synthesis but without heating reaction mixture will be a much greener way for synthesis of nanoparticles. In the present study, we compared the synthesis by using microwave heating and without heating of reaction mixture.
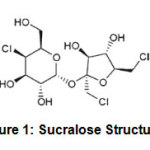 |
Figure 1: Sucralose Structure Click here to view figure |
pH can influence the reducing ability of reducing agent and particle morphology produced if the reducing agent structure can change with changing pH12. In different cases, changes in pH can affect the formation of reducing substances in a particular matrix, in this case, the decomposition of sucrose or sucralose in sugar. Decomposition or hydrolysis of sugar can run well in acidic and basic pH, with different reaction mechanisms13. Hydrolysis can be accelerated by temperature treatment, which in this case was done by heating using microwave energy. Sucralose is stable at low pH, but can be hydrolyzed at extreme pH, producing 4-chloro-deoxy-d-galactose and 1,6-dideoxy-1,6-dichloro-d-fructose, or degraded with elimination of hydrogen chloride in basic medium14.
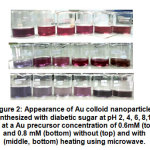 |
Figure 2: Appearance of Au colloid nanoparticles synthesized with diabetic sugar Click here to view figure |
The experimental results show that both with and without heating using microwave, Au nanoparticles can be yielded (Figure 2), although there were some slightly different characteristics. When microwave heating was used, at a higher concentration of Au precursors (0.8 mM), there is a tendency to form purple colloidal solution. This does not occur in conditions without microwave heating.
The pH range between 4 and 12 only showed a slight difference in the color of Au nanoparticle colloids in conditions without microwave heating. This indicates that the morphology of the particles produced also has similarities at various pH. At pH 2 purplish color is quite clear, which indicates the formation of particles of larger size and/or anisotropic shape. In the microwave heating treatment, the effect of pH 2 is much more noticeable, which tends to be gray and very contrast with the red color produced at pH > 2.
 |
Figure 3: FTIR spectrum of diabetic sugar (top) and AuNP synthesized by diabetic sugar (bottom) |
Figure 3 confirmed the successful of the AuNP synthesis, shown by the change of the –OH peaks in the range of 3000 – 3600 cm-1 that become less broad, sharper and well-separated peaks. This was due to the breaking of the hydrogen bonds between sucralose molecules, when treated with strong base. For sucralose solution in the diabetic sugar, the hydrogen bonds were abundant thus the peaks of –OH functional groups were very broad. The oxygen atoms have large affinity to AuNP, thus the –C-O bonds from alcohol groups were shifted to the lower wavenumber and have higher intensity due to the interaction between oxygen and AuNP.
 |
Figure 4: SPR spectra of Au nanoparticles synthesized using diabetic sugar with (top) and without (bottom) microwave heating Click here to view figure |
SPR spectra obtained at various pH showed that there was a significant wavelength and absorbance shift with a change in pH (Figures 4). This is seen more clearly in the microwave heating (Figure 4 top, left and right). In these conditions, there is a tendency that the increase in pH causes the wavelength to shift to the left (towards a smaller value), increase in absorbance and the peaks gets narrower. Shifting the wavelength towards a smaller value and narrowing the curve indicates the change in size to be relatively smaller and more uniform. Increasing absorbance shows an increase in the quantity of nanoparticles produced. However, increasing the quantity is difficult to compare quantitatively if the particle morphology is not uniform. Based on these data it can be assumed that alkaline conditions are more favorable for the formation of small, spherical and monodispersed Au nanoparticles.
Although the shift in wavelength also occured in the condition of synthesis without microwave heating, the shift is not as large as that by microwave heating (Figure 4 bottom, left and right). A fairly obvious shift is seen at pH 2, that is, the wavelength at the pH tends to be greater than at pH > 2, and with a wider peak. This indicates a larger and less uniform particle size. Almost the same as the synthesis by microwave heating, in the condition of synthesis without microwave heating, peaks at pH 10 and 12 tend to have a smaller value of wavelength compared to other pH variations.
Although the synthesis results at pH 10 and 12 tend to be similar, but when viewed from the stability, there is a clear difference for the nanoparticles synthesized at the concentration of the Au precursor of 0.8 mM, after being left for several days. Stability of Au nanoparticles as a function of pH can be seen in Figure 5. There was a change in colloidal color which was originally red to brownish yellow, which was possibly due to uncontrolled degradation as a consequnce of abundant amount of base in these conditions. At Au precursor concentrations of 0.6 mM, even this happened also at pH 10. This indicates that the higher the pH the tendency to become unstable is greater because of the increase in alkalinity. On the other hand, since NaOH base is also a strong electrolyte, increasing the base concentration can also result in increased electrostatic attraction between particles. As previously known, the increase in electrostatic attraction between particles can reduce particle stability15, so that the regulation of NaOH concentration is something that needs to be done carefully in order to obtain Au nanoparticles which have morphology as expected and are also stable until a certain time as needed.
 |
Figure 5: Au nanoparticle colloids synthesized with diabetic sugar without heating using microwave at pH 2, 4, 6, 8, 10, 12 at 0.6 mM Au precursor concentrations after several days. Click here to view figure |
Nanoparticles as catalyst for methylene blue oxidation
Methylene blue is the most important basic cationic dye, and it is the most commonly used in the textile industry. Methylene blue is such an organic dye which is resistant to many conventional waste treatment.
Au nanoparticles which has been synthesized using diabetic sugar were then applied as catalyst in the blue methylene oxidation reaction. The effectiveness of the catalyst was examined by monitoring the peaks by UV-Vis spectrophotometer. Methylene blue shows a peak in the area of around 663-665 nm. The methylene blue solution which is oxidized with hydrogen peroxide in the absence of a catalyst was used as control. In the absence of Au nanoparticle catalyst, the methylene blue oxidation reaction proceed quite slowly (Figure 6). Although there was a decrease in absorbance at a wavelength of 663 nm, the decrease was not too large, even up to 5 hours. This indicates that the reaction requires a catalyst that can accelerate the reaction process.
Au nanoparticles in the solution phase have a very small concentration, so if applied directly as a catalyst it will not give good performance. Therefore it is necessary to purify Au nanoparticles by centrifugation at a certain agitation speed until the solid phase is obtained. The precursor concentration of the Au used was 1.0 mM, to get more concentrated Au nanoparticles. The pH was adjusted around 10-11, using NaOH. The addition of electrolytes in sufficient quantities also plays a role in facilitating the formation of solids of Au nanoparticles.
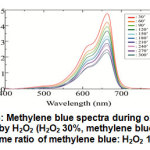 |
Figure 6: Methylene blue spectra during oxidation reaction by H2O2 (H2O2 30%, methylene blue 4 ppm, volume ratio of methylene blue: H2O2 10: 1) Click here to view figure |
It is known that at very small particle sizes (<10 nm) Au nanoparticles have very good catalytic activity. The catalytic activity is influenced by several factors including particle size and shape16. Since the synthesis of Au nanoparticles with diabetic sugar is relatively easy, simple, does not require a long time and does not involve synthetic chemicals, the Au nanoparticle catalyst synthesized with diabetic sugar is very potential to be applied to various reactions, including methylene oxidation reaction by hydrogen peroxide.
 |
Figure 7: The appearance of a methylene blue solution after undergoing an oxidation reaction catalyzed by Au nanoparticles for 1 hour at various volumes of methylene blue Click here to view figure |
The results indicate that the addition of AuNP in a mixture of methylene blue and hydrogen peroxide can significantly increase the reaction rate, depending on the volume of methylene blue and the ratio of AuNP : methylene blue. Figure 7 shows that at volume of methylene blue of 5 mL, after 1 hour the mixture of AuNP-methylene blue-H2O2 undergoes a color change from blue to clear, indicates that the oxidation of methylene blue occurred significantly. The intensity of blue fading decreases as the volume of methylene blue increases, due to a decrease in the ratio of AuNP catalysts: methylene blue. The role of the catalyst in this case is to provide a surface where the interaction between methylene blue and H2O2 molecules occurred intensively so that the collision between methylene blue and H2O2 is more likely to occur and the reaction is easier to proceed. The lower volume ratio of AuNP : methylene blue can be interpreted as low amount of AuNP for the same volume of methylene blue, and this results in a lower surface area available for the reaction, so the reaction runs slower. At the same time, fading blue becomes even less intense under these conditions.
Optimization of the experiment condition needs to be done to find out the best conditions to produce the highest amount of catalysis products or the highest effectiveness of the catalysis. The parameters examined in this experiment included the weight of AuNP, volume of methylene blue, and volume of H2O2. The stability of AuNP, kinetics of the reaction, and the phenomenon of reactions that occured are also studied in this experiment.
Although pH may played a role in the efficiency of the catalysis, the experiment was not conducted using buffered solution. Previous literature reported that the oxidation of methylene blue was more significant for unbuffered solution than for buffered solution17.
Effect of AuNP mass
Efficiency of Au nanoparticle catalysis was studied in various mass variations namely 0.05; 0.10; 0.15; 0.20; and 0.25 grams, in a reaction time of 30 minutes. Significant decrease in absorbance and increase in efficiency were seen with increasing mass of nanoparticles (Figure 8). The increased efficiency of catalysis is caused by the greater size of the surface area needed to interact with the reactants involved in the reaction.
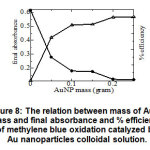 |
Figure 8: The relation between mass of AuNP mass and final absorbance and % efficiency of methylene blue oxidation catalyzed by Au nanoparticles colloidal solution. Click here to view figure |
Effect of volume of methylene blue solution
The volume of methylene blue solution may affect the efficiency of the oxidation reaction, thus it varied while kept the AuNP mass and other parameters constant. At volume of methylene blue 10 mL (AuNP mass of 0.10 g, H2O2 1 mL), after adding AuNP to the methylene blue solution, the blue color changes readily, indicating a oxidation reaction took place immediately. The purple color emerged instead of clear solution, which suggested that the AuNPs were redissolved back into the colloidal solution. As shown in Figure 9 (top), the characteristic peak of methylene blue solution was absence, instead, the specific SPR peak of AuNPs were formed, at all various reaction time. By deconvolution technique to analyze the spectra, it was found that the peak of methylene blue was not gone completely, though the absorbance were very low (about 0.04 after 60 minutes). When the volume of methylene blue was increased to 20 mL, it is shown that there is a significant decrease in peak at a wavelength of 663 nm (i.e. wavelength that is characteristic for methylene blue) over time. This indicates the occurrence of a methylene oxidation reaction to produce a new product, resulting in a decrease in the concentration of methylene blue. After 30 minutes, the peak at 663 nm disappears, and only the shoulder remains. Similar as obtained for volume of methylene blue of 10 mL, new peaks of AuNPs were formed at around 550 nm. When the curves were deconvoluted using Gaussian fitting program, it can be seen that the peak around 660 nm – which is assumed to be methylene blue – was still present, but with very low absorbance (Figure 9 bottom, right). But the shift of the peak towards a lower value can also be assumed as the peak of the large AuNP caused by an interaction with oxidized species of methylene blue. The curve for AuNP also has a low absorbance, since the solids which are used only in small quantities, thus the initial absorbance is also low. Based on the synthesis results, initial absorbance of the as-formed AuNP colloid was only about 0.2 to 0.3, prior to separation of solid phase from the colloidal solution. In this experiment, the absorbance of the AuNP peak may decrease from its initial value due to the influence of the oxidation reaction.
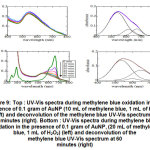 |
Figure 9: Top : UV-Vis spectra during methylene blue oxidation in the presence of 0.1 gram of AuNP (10 mL of methylene blue, Click here to view figure |
Further examination was carried out by applying control which is a solution of methylene blue added with AuNP in the absence of H2O2. If the decrease in absorbance occurs significantly, then AuNP do not only act as catalysts but also as adsorbents. Figure 10 (top) showed that AuNP added to methylene blue solution – in the absence of H2O2 – did not produce significant absorbance changes, shown by an almost flat efficiency curve, and based on calculations the efficiency of the catalysis was only 23.56% (Table 1). On the other hand, the addition of H2O2 in methylene blue solution in the absence of AuNP produced very little decrease, at the same time, which was only 22.20%. Based on this data, it can be concluded that AuNP do not act as adsorbents, but only as catalysts. The decrease in absorbance of AuNP as described earlier may be due to the interaction with oxidized methylene blue molecules leading to the less effective interactions between stabilizers and AuNP. AuNP that are not coated by stabilizers are relatively easy to aggregate and their size becomes larger. With greater size, the absorbance of SPR spectra of AuNP which are characteristic at around 540 nm will be reduced.
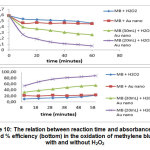 |
Figure 10: The relation between reaction time and absorbance (top) and % efficiency (bottom) in the oxidation of methylene blue, with and without H2O2 Click here to view figure |
Table 1: % efficiency of methylene blue oxidation, with and without AuNP and H2O2, as function of reaction time
|
Time (minutes) |
% efficiency | |||
| MB + H2O2 | MB + Au nanoparticles | MB (20 mL) + H2O2 + Au nanoparticles |
MB (30 mL) + H2O2 + Au nanoparticles |
|
|
10 |
9.66 | 21.36 | 32.54 | 54.41 |
| 20 | 11.86 | 20.00 | 38.47 |
67.80 |
|
30 |
13.39 | 22.20 | 43.22 | 76.61 |
| 40 | 14.75 | 21.19 | 48.64 |
81.53 |
|
50 |
16.95 | 22.71 | 52.88 | 84.24 |
| 60 | 22.20 | 23.56 | 55.59 |
88.14 |
Kinetics of Methylene Blue Oxidation
At constant Au nanoparticles mass and H2O2 concentration, the rate equation can be expressed as :
![]()
Where kobs represent the pseudo-nth-order rate constant of the reaction. The absorbance versus time is measured at λ 664 nm.
The above equation can be integrated to :
![]()
Based on the results of the kinetics experiment, the reaction followed pseudo first order (assuming the large excess of H2O2) since it gives linear plot of ln [(At-A~)/(A0-A~)] vs time (Figure 11). This is in accordance with what has been learned by2 regarding the methylene blue oxidation reaction catalyzed by dendrimer encapsulated silver and gold nanoparticles.
![Figure 11: Plot between ln [(At-A~)/(A0-A~)] vs time at condition : 0.1 g Au nanoparticles, 20 mL methylene blue, 1 mL H2O2](http://www.orientjchem.org/wp-content/uploads/2019/10/Vol35_No5_Cat_Nur_fig11-150x150.jpg) |
Figure 11: Plot between ln [(At-A~)/(A0-A~)] vs time at condition : 0.1 g Au nanoparticles, 20 mL methylene blue, 1 mL H2O2 Click here to view figure |
Hydrogen peroxide acts as an oxidizing agent in the methylene blue oxidation reaction. Since hydrogen peroxide is a reactant in this reaction, it must be analyzed for its effect on the oxidation effectiveness. Although it showed a decrease in absorbance with an increase in the amount of H2O2, the decrease was not too large (Figure 12).
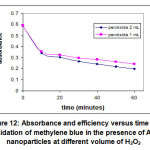 |
Figure 12: Absorbance and efficiency versus time for oxidation of methylene blue in the presence of Au nanoparticles at different volume of H2O2 Click here to view figure |
The effect of the amount of H2O2 on the rate of methylene blue oxidation reaction was also studied in terms of kinetics. Both at volume of H2O2 of 1 mL and 2 mL the reactions followed pseudo first order with R2 values that were close to 1 (Figure 13). The difference between the two reactions were in the value of kobs (rate constant), in which the value of kobs for H2O2 of 2 mL was greater than that of 1 mL (Table 2).
![Figure 13: Plot between ln [(At-A~)/(A0-A~)] vs time at volume H2O2 of 1 mL (bottom) and 2 mL (top). The experimental condition : 0.1 g AuNP, 20 mL methylene blue](http://www.orientjchem.org/wp-content/uploads/2019/10/Vol35_No5_Cat_Nur_fig13-150x150.jpg) |
Figure 13: Plot between ln [(At-A~)/(A0-A~)] vs time at volume H2O2 of 1 mL (bottom) and 2 mL (top). The experimental condition : 0.1 g AuNP, 20 mL methylene blue Click here to view figure |
Stability of the AuNP
AuNPs which are fresh and have been stored for up to 3 days showed differences catalytic activity. Freshly synthesized nanoparticles have greater efficiency than those that have been stored for 3 days, judging from the large decrease in absorbance of methylene blue. For samples with 3 days old, with a weight of 0.2 grams of AuNP, an efficiency of 82.7% can be obtained after 180 minutes, while for fresh samples with only 0.1 gram AuNP, an efficiency of 88.13% has been achieved only in the 60 minutes (Table 2). For samples with 3 days old, after 180 minute, the methylene blue UV-Vis spectrum still exhibit double peaks in the area above 600 nm like its initial shape, but with lower absorbance (Figure 14). The AuNP peaks were not present in this spectra, assumed that they were not stable and could not redispersed into colloidal solution. The phenomenon means the stability of the AuNP closely related with the catalytic activity or the efficiency of catalysis reaction.
Table 2: The kinetic parameters of methylene blue oxidation in the presence of AuNP catalyst for fresh and third day sample
|
AuNP mass (gram) |
Volume of methylene blue (mL) | Volume of H2O2 (mL) | R2 | kobs (min-1) | % efficiency*1 | sample condition |
| 0.2 | 20 | 1 | 0.9962 | 0.0074 | 43.89 |
third day |
|
0.2 |
20 | 2 | 0.9973 | 0.0109 | 66.44 | third day |
| 0.1 | 20 | 1 | 0.9987 | 0.0597 | 88.13*2 |
fresh |
*1after 1 hour
*2assumed that the second peak at 646 nm was from methylene blue
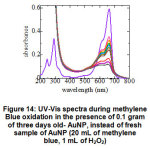 |
Figure 14: UV-Vis spectra during methylene blue oxidation in the presence of 0.1 gram of three days old- AuNP, instead of fresh sample of AuNP (20 mL of methylene blue, 1 mL of H2O2) Click here to view figure |
Conclusion
Au nanoparticles can be synthesized in a simple, easy, and environmentally friendly step by using diabetic sugar under the following optimum conditions: sugar concentration of 15% -20%, 0.60 – 0.80 mM Au precursor concentration and pH 11. Solid AuNP which have been purified and separated from the solution phase, can be applied as a catalyst in the methylene blue oxidation reaction by H2O2. The kinetic of methylene blue oxidation reaction followed pseudo first order with the rate constant depends on the characteristic of AuNP and experimental condition.
Acknowledgements
This work was financially supported by Polytechnic of AKA Bogor. We acknowledge the Environmental Laboratory and Physics and Instrumentation Laboratory of Polytechnic of AKA Bogor for supplying the tool.
Conflict of Interest
The authors declare no conflicts of interest.
References
- Haruta, M., Gold as a novel catalyst in the 21st century: preparation,working mechanism and applications, Gold Bull. 37, 27–36. 2004.
- Ali K. Ilunga and Reinout Meijboom. Catalytic Oxidation of Methylene Blue by Dendrimer Encapsulated Silver and Gold Nanoparticles. Journal of Molecular Catalysis, Elsevier. 48-60.2016.
- Chen, T., Ba-Tian Chen, Konstantin V. Bukhryakov, Valentin O. Rodionov Thiols make for better catalysts: Au nanoparticles supported on functional SBA-15 for catalysis of Ullmann-type homocouplings, Chem. Commun., 53, 11638-11641. 2017.
- Zhang, H., Xiangong Deng, Chengpeng Jiao, Lilin Lu, Shaowei Zhang, Preparation and catalytic activities for H2O2 decomposition of Rh/Au bimetallic nanoparticles, Materials Research Bulletin 79, 29–35. 2016.
- Sugano Y., Y. Shiraishi, D. Tsukamoto, S. Ichikawa, S. Tanaka, T. Hirai, Supported Au-Cu Bimetallic Alloys Nanoparticles : An Aerobic Oxidation Catalyst with Regenerable Activity by Visible-Light Irradiation, Angew Chem Int Ed Engl. 52, 5295-5299.2013
- M.S Chen and D.W. Goodman. Structure-activity Relationship in Supported Au Catalysis . Catalysis Today. Elsevier, Volume 111, Issues 1-2, pp 22-33. 2006
- Britt Hvolbae, Ton V.W Janssens, Bjerne S. Clausen, Hanne Falsig, Claus H. Christensen, Jens K. Norskov. Catalytic Activity of Au Nanoparticles. Nanotoday. Volume 2, Issue 4, pp 14-18. 2007
- Sudipa Panigrahi, Subrata Kundu,Sujit Ghosh, Sudip Nath, Tarasankar Pal. General Method of Synthesis for Metal Nanoparticles. Journal of Nanoparticle Research. Issue 4, pp 411-414. 2004.
- Pettegrew, C., Zheng Dong,M. Zubayed Muhi, Scott Pease, M. Abdul Mottaleb, and M. Rafiq Islam, Silver Nanoparticle Synthesis Using Monosaccharides and Their Growth Inhibitory Activity against Gram-Negative and Positive Bacteria, ISRN Nanotechnology, Article ID 480284, 8 pages. 2014
- Meshram, S.M., Shital R. Bonde, Indarchand R. Gupta, Aniket K. Gade, Mahendra K. Rai, Green synthesis of silver nanoparticles using white sugar, IET Nanobiotechnol., pp. 1–5,doi: 10.1049/iet-nbt.2012.0002. 2013
- Sharma, S.; Pallab S., Arun C., Siddhartha S. G., Fabrication of antibacterial silver nanoparticle—sodium alginate–chitosan composite films, RSC Adv., 2, 5837-5843. 2012
- Foliatini, Yoki Yulizar, Mas Ayu Elita Hafizah, The synthesis of alginate-capped silver nanoparticles under microwave irradiation, Math.Fund. Sci., 47 (1) 32-51. 2015
- Edye, L.A. An Overview Of Sucrose Degradation, Proc. Int. Soc. Sugar Cane Technol., 24: 353-355. 2001.
- Glória, M.B.A., in Encyclopedia of Food Sciences and Nutrition, Second Edition.2003
- Lu, K., Theoretical analysis of colloidal interaction energy in nanoparticle suspensions, Ceramics International, 34, 1353–1360. 2008
- Cortie, M. B.; E. Van der lingen. Catalytic gold nano-particles, Materials Forum, 26, 1-14. 2002
- Matumuene Joe Ndolomingo and Reinout Meijboom. Kinetic Analysis of Catalytic Oxidation of Methylene Blue Over γ-Al2O3 Supported Copper Nanoparticles. Applied Catalysis A : General, pp 33-43. 2015

This work is licensed under a Creative Commons Attribution 4.0 International License.










