Synthesis of Fluoroquinolones Derivatives as Antimicrobial Agents
Lubna Swellmeen*1, Amal Uzrail2, Rand Shahin3 and Yusuf AL-Hiari4
1Department of Pharmaceutical Sciences, Faculty of Pharmacy, Zarqa University Jordan.
2Department of Medical Analysis, Faculty of Allied Medical Sciences, Zarqa University Jordan.
3Department of Pharmaceutical Chemistry, Faculty of Pharmaceutical Sciences, Hashemite University Jordan.
4Faculty of Pharmacy, The University of Jordan, Amman 11942, Jordan Zarqa, Jordan.
Corresponding Author E-mail: lswellmeen@zu.edu.jo
DOI : http://dx.doi.org/10.13005/ojc/350401
Article Received on : 08-04-2019
Article Accepted on : 05-08-2019
Article Published : 19 Aug 2019
Fluoroquinolones are well known to have an anti-infective action. In the present study we described the synthesis of novel florouquinolones derivative as antimicrobial agent. The biological test highlighted a good inhibitory activity for the 7-Chloro-1-Alkyl-6-fluoro-8-nitro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid derived synthons especially against pathogenic Gram-negative bacteria (Pseudomonas aeruginosa) and Gram-positive bacteria (Staphylococcus aureus and Streptococcus agalactiae). The binding interactions were monitored and could explain the good inhibitory activity of the synthesized derivatives of florouquinolones.
KEYWORDS:Antibacterial activity; Florouquinolones derivatives; Gram-negative bacteria; Gram-positive bacteria
Download this article as:| Copy the following to cite this article: Swellmeen L, Uzrail A, Shahin R, AL-Hiari Y. Synthesis of Fluoroquinolones Derivatives as Antimicrobial Agents. Orient J Chem 2019;35(4). |
| Copy the following to cite this URL: Swellmeen L, Uzrail A, Shahin R, AL-Hiari Y. Synthesis of Fluoroquinolones Derivatives as Antimicrobial Agents. Orient J Chem 2019;35(4). Available from: http://www.orientjchem.org/?p=59148 |
Introduction
Anti-infective agents played a major role in saving human lives. Among these agents are the fluoroquinolones class, which had risen to be highly appreciated, especially if there was microbial resistance against penicillin and macrolide. Fluoroquinolone pharmacophore (Figure 1) is well known to have antibacterial activity, and since 1980 there were many generation introduced to the market [1]. The fluoroquinolones were found to be effective to combat urinary tract infection [1] ideal in treating Neisseria gonorrhea [1] and highly effective to treat tuberculosis [2]. In addition, fluoroquinolone nucleus are presented widely in biologically active compounds such as PDE 4 inhibitors [3], PIM kinase inhibitors [4], GSK β inhibitors [5].
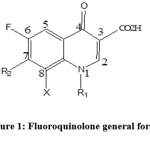 |
Figure 1: Fluoroquinolone general formula. |
The encouraging properties of fluoroquinolones such as; broad spectrum of activity, good oral bioavailability, good tissue penetrability and low incidence of adverse effect [6] gave them high appreciation and encouraged researcher to investigate their usefulness as a source of potent antibacterial drugs.
In our previous work, we have described various modifications to the main structure of fluoroquinolone, including the introduction of different substituent’s at position 1 and 7 (Figure 1), and in continuation for obtaining a new fluoroquinolone derivatives with excellent antibacterial activity, our team reported synthon C (1,2,3and 4) as a potent antibacterial agents.
Materials and Methods
Experimental
Molecular modelling
Computational software
The following software packages were utilized:
CS ChemDraw Ultra 6.0, Cambridge Soft Corp. (http:// www.cambridgesoft.Com), USA.2D Structure drawing was performed employing.
Discovery Studio 4.5 (DS 4.5) Standalone Applications, including docking Biovia ® (www.3ds.com), USA.
Accelrys Enterprise Platform Server (AEP) (www.accelrys.com), USA.
The crystal structures of gyrase enzyme were obtained from the protein data bank (http://www.rcsb.org/).
Molecular modelling studies
We docked our synthesized molecules using the Dock Ligands (LibDock) docking alogarithim implemented in the DS 4.5 into the binding pocket of the successful DNA gyraseenzyme namely : (PDB code: 5L3J, resolution 2.83 Ǻ).
Chemistry
General
All chemicals, reagents and solvents were of analytical/ synthetic grade that purchased from Sigma-Aldrich and Acros, Belgium, and used directly without further purification.Nuclear magnetic resonance spectra (NMR) were recorded on Bruker, Avance DPX-300 spectrometer.
High-resolution mass spectra (HRMS) were measured in positive or negative ion mode using electrospray ion trap (ESI) technique by collision induced dissociation on a Bruker APEX-4 (7 Tesla) instrument. Melting points (MP) were determined in open capillaries on a Stuart scientific electro-thermal melting point apparatus, and are uncorrected. Infra red (IR) spectra were recorded using Shimadzu 8400F FT-IR spectrophotometer (KBr discs). Microanalyses were performed using EuroVector Elemental Analyser, model (EA3000 A), Jordan University.
Synthesis of synthon (A)
The synthesisof 7-Chloro-1-Alkyl-6-fluoro-8-nitro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid was previously described by our group [4, 7, 8]. Then adding a substitution at position 7 of (a and b) synthons, was prepared according to reported method [4, 8] provided the nitro derivatives synthon C (Scheme 1). The synthesized compounds gave satisfactory analytical and spectroscopic data in accordance with their depicted structures.
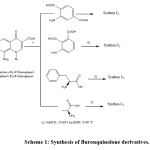 |
Scheme 1: Synthesis of flurouquinolone derivatives Click here to view scheme |
2-[(3-carboxy-1-(4-fluorophenyl)-6-fluoro-8-nitro-4-oxo-1,4-dihydroquinolin-7-yl)amino]terephthalic acid (C1)
2-Aminoterphthalic acid (3.2 g, 18mmol) was reacted with synthon a (2.0g, 5.26mmol) and dimethyl sulfoxide (DMSO) 40ml and pyridine 10ml was heated at 70 °C for 10 days under reflux conditions. The mixture was left to cool, then pH was adjusted by 3.5N HCl dried to give the title compound as dark brown solid; Yield ≈ 1.6 g (60%); mp = 264 °C; 1H- NMR (300 MHz,DMSO-d6): δ 7.00 (d, 2H, H-2”,, H-6”), 7.02 (d, 2H, , H-3”,H-5”), 7.77-8.1 (m, 3H, ArH), 8.46 (d, 1H, H-5), 8.90 ( s, 1H, H-2), 9.24 (br s, 1H, NH),13.50 – 15.40 (2 br s, C(3)COOH and C(2′)COOH);IR (NaCl): ν 3417, 2067, 1701, 1643, 1265 cm1; Anal.Calcd. for C24H13F2N3O9 (525.06), C, 54.87; H, 2.49; F, 7.23; N, 8.0; Found: C, 54.77,H, 2.43; N, 7.03.
2-[(3-carboxy-1-(4-fluorophenyl)-6-fluoro-8-nitro-4-oxo-1,4-dihydroquinolin-7-yl)amino] phthalic acid (C2)
A stirred mixture of 2-Aminophthalic acid (1.6 g, 9mmol), synthon a (1.0g, 2.63 mmol) and DMSO 20ml and pyridine 5ml was heated at 70 °C for 10 days under reflux conditions. The same procedure carried out as with synthon C1was done and yielded brownish solid compound ; Yield ≈ 0.8 g (60%); mp=260 °C; 1H- NMR (300MHz, DMSO-d6 ): δ 7.04-7.11(m, 4H, H-2”, H-3”, H-5”, H-6”), 7.94-8.09 (m, 3H, ArH), 8.11 (d, 3JH-F = 9 Hz, 1H, H-5), 8.90 ( m, 1H, H-2), 9.15 (br s, 1H, NH); IR (NaCl): ν 3417, 2067, 1701, 1643, 1265 cm1; Anal. Calcd. for C24H13F2N3O9 (525.06), C, 54.87; H, 2.49; F, 7.23; N, 8.0; Found: C, 54.77, H, 2.43; N, 7.03.
7-[(2-carboxy-1-phenylethyl)amino]-1-(4-fluorophenyl)-6-fluoro-8-nitro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (C3)
A stirred mixture of 3-phenyl β-alanine (4.06 g, 24mmol), synthon a(2.0 g, 5.2mmol) and sodium hydrogen carbonate (3.0 g, 36mmol) in 50 % aqueous ethanol (280mL) was heated at 70-80 °C for 6 days under reflux condition. The mixture was worked up as described for synthon C1. Yellow solid was collected; Yield 2.2 g (74.4%); mp: 280°C; 1H- NMR (300MHz, DMSO-d6 ): δ 2.76 (br m, 2H, CH2-COOH ,4.31 (br s, 1H, CH-NH ), 7.10-7.21 (4H, P-fluorophenyl), 7.46-7.55 ( br m,5H, ArH), 7.86 (d, 3JH-F = 14.1 Hz, 1H, H-5), 8.36 (s, 1H, H-2), 15.70(br s, 2H, 2 COOH); IR (NaCl): υ 3417, 2098, 1643, 1481, 1411, 1319, 1188, 1010 cm-1; Anal. calcd. for C25H17F2N3O7 (509.1): C, 58.94; H, 3.36; N, 8.25. Found: C, 58.94; H, 3.36; N, 8.25.1.H, 3.36; N, 8.25.
7-(2-Carboxy-ethylamino)-1-(4-chloro-phenyl)-6-fluoro-8-nitro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid .(C4)
quinoline-3-carboxylic acid .(C4)
A mixture of b-alanine (1.05 g, 11.5 mmol), Synthon b (1.0 g, 2.2mmol) and sodium hydrogen carbonate (3 g, 35.8 mmol) in 50% aqueous ethanol (120 mL) was heated for 6 days under reflux conditions. The product wasworked up as described for synthon C1 and yielded a yellowish color. 1H NMR (300 MHz, DMSO- d6): δ2.28 (d, J = 8.1, 2H,CH2-COOH), 3.66 (m, 2H, CH2-NH), 7.51 (br t, J = 5.7 Hz, 1H, NHCH2),7.51–7.54 (m, 2H, H-3′, H-5′), 7.61–7.67 (m, 2H, H-2′, H-6′),8.2 (H-5), 8.62 (s, 1H, H-2); HRMS (ESI,_ve): m/z [M_H]_448.04 C19H12ClFN3O7 requires 448.0353.
Biological Evaluation
Test microorganisms
Six pathogenic bacterial strains were used in the antimicrobial assays, four Gram-positive (Staphylococcus aureus ATCC29213, Staphylococcus saprophyticus ATCCRBAA 750 ,Streptococcus agalactiae ATCC13813,Streptococcus pyogenes ATCC19615, and two Gram-negative (Pseudomonas aeruginosa ATCC27853, Escherichia coli ATCC11775). Those pathogens were chosen based on their clinical and pharmacological importance. Antibacterial activities were evaluated by the agar well diffusion method as recommended by the Clinical and Laboratory Standards Institute (CLSI) [9-11] and the European Committee on Antimicrobial Susceptibility Testing (EUCAST)[12-13].
Measurement of antibacterial activity of the synthetic compounds
Preparation of synthetic compounds for microbiological assay
Stock solutions of 20 and 30 mg of each synthetic compound dissolved in 1mL of dimethyl sulfoxide (DMSO), as solvent. They were sterilized by filtration, and stored at 4oC. The antimicrobial activity of the synthesized compounds was evaluated by the agar well diffusion method [14].
Determination of antibacterial activity by agar well diffusion method
All the synthetic compounds of different concentrations were screened for their antibacterial activities against the Staphylococcus aureus, Staphylococcus saprophyticus, Streptococcus agalactiae, Streptococcus pyogenes, Pseudomonas aeruginosa and Escherichia coli by agar well diffusion assay. Isolated pure colonies from fresh grown bacteria were transferred from the plates into sterile normal saline solution and vortexed to form bacterial homogenous suspensions. The turbidity was then adjusted to 0.5 McFarland standard units, and a volume of the inoculum was spread on the entire surface of agar. Then, a hole with a diameter of 6-8 mm was punched aseptically using a sterile cork borer, and a volume (20-100 μl) of the synthetic compound was introduced at the desired concentration into the well. Control experiments were carried out under similar conditions using amoxicillin (20 mg), ciprofloxacin (5 mg) and gentamicin (10 mg), as positive controls, and sterile distilled water as negative control. The zones of growth inhibition were measured in millimeters (mm) after 18-24 hours of incubation at 37oC. The sensitivities of the microorganisms to the synthetic compounds were determined by measuring the sizes of inhibitory zones, and values <8 mm were considered as being not active against the tested bacterial strains [15].
Results and Discussion
The synthesized derivatives have been obtained in a good yield, and showed good antibacterial activity against both gram positive and gram negative bacteria (Table 1), and it’s well known that position 7 modifications can bring about the major changes in potency. Attachment of aromatic rings having an amino substitution results in improved activity and it also affects the pharmacokinetics of the compound [16].
Table 1: The antibacterial activity of prepared synthetic compounds and standard drugs against bacterial testing strains.
| Zone of inhibition (mm) | |||||||
| Compound | Concentration(mg/ml) | E.coli | P.aeruginosa | Staph.aureus | Staph. saprophyticus | Strep. agalactiae | Strep. pyogenes |
| C1 | 20 | 17 | 22 | 23 | 12 | 15 | 23 |
| 30 | 20 | 17 | 20 | 17 | 23 | 26 | |
| C2 | 20 | 18 | – | 23 | 16 | 14 | 25 |
| 30 | 21 | – | 20 | 17 | 22 | 21 | |
| C3 | 20 | 20 | 20 | 19 | 18 | 20 | 23 |
| 30 | 20 | 20 | 18 | 12 | 17 | 20 | |
| C4 | 20 | 22 | 22 | 25 | – | – | – |
| 30 | – | – | 13 | 10 | 19 | 20 | |
| Amoxacillin (20 mg)(+ve) | 31 | 20 | 20 | 25 | 19 | 30 | |
| Ciprofloxacin (5 mg)(+ve) | 20 | 16 | 20 | 26 | 25 | 22 | |
| Sterile D.W.(-ve) | – | – | – | – | – | – | |
E.: Escherichia, Staph. Staphylo coccus, P.: Pseudomonas, Strep.: Streptococcus, (-):no inhibition, (+ve): positive control, (-ve): negative control, D.W.: distilled water.
The lipophilic substitution at position 1 led to synthons with a good activity against gram positive bacteria (Figure 2), and position 7 substitution with 2-aminophthalic acid and 3-aminoterphthalic acid imparted both gram negative and gram positive activity(Figure 2).
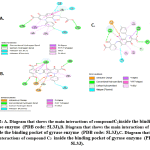 |
Figure 2: A. Diagram that shows the main interactions of compound C1 inside the bindingpocket of gyrase enzyme (PDB code: 5L3J), Click here to view figure |
B. Diagram that shows the main interactions of compound C2 inside the binding pocket of gyrase enzyme (PDB code: 5L3J), C. Diagram that shows the main interactions of compound C3 inside the binding pocket of gyrase enzyme (PDB code: 5L3J).
Conclusions
In summary, we have efficiently synthesized a novel series of fluoroquinolone-modified analogues. Biological testing showed that some of the derivatives have good antibacterial activity in a series of primary assays. .
Acknowledgements
This project was funded by the deanship of research in Zarqa University/Jordan, (1/1/307). The authors thank the faculty of pharmacy in Zarqa University for their generous help and support.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper
References
- Sharma PC., Janin A, Jain S. Fluoroquinolone antibacterials: A review on chemistry, microbiology and therapeutic prospects. Acta Poloniae Pharmaceutica ñ Drug Research 2009; 66(6): 587-604.
- Ginsburg AS., Grosset JH, BishaiWR.. Fluoroquinolones, tuberculosis and resistance. Lancet Infect. Dis. 2003; 3(7): 432-42.
- Lunniss CJ1, Cooper AW, Eldred CD, Kranz M, Lindvall M, Lucas FS, Neu M, Preston AG, Ranshaw LE, Redgrave AJ, Ed Robinson J, Shipley TJ, Solanke YE, Somers DO, Wiseman JO. Quinolines as a novel structural class of potent and selective PDE4 inhibitors: Optimisation for oral administration. Bioorganic & Medicinal Chemistry Letters 2009; 19 (5): 1380–1385.
- Swellmeen L., Shahin R., Al-Hiari Y., Alamiri A, Hasan A,, Shaheen O.. Structure based drug design of Pim-1 kinase followed by pharmacophore guided synthesis of quinolone-based inhibitors. Bioorganic & Medicinal Chemistry 2017; 25 (17): 4855–4875.
- Cociorva OM1, Li B, Nomanbhoy T, Li Q, Nakamura A, Nakamura K, Nomura M, Okada K, Seto S, Yumoto K, Liyanage M, Zhang MC, Aban A, Leen B, Szardenings AK, Rosenblum JS, Kozarich JW, Kohno Y, ShrederKR. Synthesis and structure–activity relationship of 4-quinolone-3-carboxylic acid based inhibitors of glycogen synthase kinase-3b. Bioorganic & Medicinal Chemistry Letters 2011; 21(19): 5948–5951.
- Blondeau JM. Expanded activity and utility of the new fluoroquinolones: a review ClinTher 1999; 21(1):3-40.
- Al-Hiari YM, Abu-Dahab R, El-Abadelah MM. Heterocycles [h]-fused onto 4-oxoquinoline-3-carboxylic acid, part VIII. Convenient synthesis and antimicrobial properties of substituted hexahydro[1,4]diazepino[2,3-h]quinoline-9-carboxylic acid and its tetrahydroquino[7,8-b]benzodiazepine analog . Molecule 2008; 13(11):2880-93.
- Al-Hiari YM, Al-Rajab W, Shakya AK, AbdelRahim E, AlZweiri MH, Rustam L, Darwish R. Antimicrobial screening of novel N-4-fluorophenylquino-[7,8-b][1,4]-benzodiazepin-3-carboxylic acid derivatives Rev. Roum chim 2015;60(9): 899–905.
- Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). Method for the determination of Broth dilution MICs of antifungal agents for fermentative yeasts. ClinMicrobiol Infect Diseases. 2008; 14(4):398–405.
- CLSI. Reference Method for Broth Dilution Antifungal SusceptibilityTesting of Yeasts; Approved Standard-Third Edition; CLSI Document M27-A3. Wayne, PA: Clinical and Laboratory Standards Institute; 2008.
- CLSI. Reference Method for Broth Dilution Antifungal SusceptibilityTesting of Filamentous Fungi; Approved Standard CLSI Document M38-A2. Wayne, PA: Clinical and Laboratory Standards Institute; 2008.
- Chryssanthou E, Cuenca-Estrella M. Comparison of the EUCAST-AFSTbroth dilution method with the CLSI reference broth dilution method (M38-A) for susceptibility testing of posaconazole and voriconazole against Aspergillus spp. ClinMicrobiol Infect 2006; 12(9):901–904.
- Cuenca-Estrella M, Moore CB, Barchiesi F, et al. Multicenter evaluation of the reproducibility of the proposed antifungal susceptibility testing method for fermentative yeasts of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antimicrobial Susceptibility Testing (AFST-EUCAST). ClinMicrobiol Infect. 2003; 9(6):467–474.
- Esmadi FT, Khabour OF, Albarqawi AI, Ababneh M, Al-Talib M. Synthesis and characterization of some transition metal complexes of thiocarbohydrazone Schiff bases. Jordan J Chem. 2013; 8(1):31–43.
- Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008; 3(2):163–175.
- Tillotson GS: J. Med. Microbiol. 44, 320 (1996). Quinolones: structure-activity relationships and future predictions.

This work is licensed under a Creative Commons Attribution 4.0 International License.









