Synthesis, Characterization and Evaluate the Biological Activity of Novel Heterocyclic Derivatives from Azomethine Compounds
Rasim Farraj Muslim1 and Suheb Eaid Saleh2
1Department of Ecology, College of Applied Sciences-Hit, University Of Anbar, Anbar, Iraq.
2Directorate of education Anbar, Ministry of eduction, Anbar, Iraq.
Corresponding Author E-mail: nsquiming@up.edu.ph
DOI : http://dx.doi.org/10.13005/ojc/350416
Article Received on : 22-05-2019
Article Accepted on : 06-07-2019
Article Published : 16 Aug 2019
This research includes synthesis of new seventh-membered heterocyclic derivatives as 1,3-oxazepine-dione derived from azomethine compounds. Azomethine compounds R1-R4 were synthesized by the reaction of aromatic aldehydes with primary aromatic amines. The novel of 1,3-oxazepine-dione derivatives R5-R9 were obtained from the treatment of azomethine compounds with anhydrides. The synthesized compounds were checked by TLC technique, spectral methods (FT-IR, H1-NMR) and measurements of some its physical properties. The biological activity of the heterocyclic derivatives was investigated against bacteria and fungi in vitro.
KEYWORDS:Azomethine; anti-fungal; anti-bacterial; 1,3-oxazepine
Download this article as:| Copy the following to cite this article: Muslim R. F, Saleh S. E. Synthesis, Characterization and Evaluate the Biological Activity of Novel Heterocyclic Derivatives from Azomethine Compounds. Orient J Chem 2019;35(4). |
| Copy the following to cite this URL: Muslim R. F, Saleh S. E. Synthesis, Characterization and Evaluate the Biological Activity of Novel Heterocyclic Derivatives from Azomethine Compounds. Orient J Chem 2019;35(4). Available from: https://bit.ly/2TAdHqK |
Introductıon
Azomethine compounds are the compounds containing (-HC=N-) group, the best and easiest method of azomethine synthesis is the condensation reaction between the carbonyl group (C=O) of ketones or aldehydes and the amino group (NH2) of primary amines [1-4]. The equivalent rate 2:1 of 3- bromo benzaldehyde and 1,3- diamino propan- 2- ol gave the azomethine compound described in the following reaction [5].
Oxazepines are heterocyclic compounds of the seven-membered ring with two heteroatoms (O and N), the oxygen atom is located at position 1 and a nitrogen atom in positions -2, -3 or -4 [1]. To a solution of 0.01 mole of azomethine in dry toluene, a solution of maleic anhydride 0.02 mole in ethanolic solution was added dropwise with stirring then refluxed for 3-4 hours [6]. The reaction of the following azomethine compound with phthalic anhydride in dry benzene gave substituted oxazepine derivatives [7].
In this study, the synthesized compounds have been proven by TLC techneque and melting point assay. Their structures have been characterized by 1H-NMR and FT-IR spectroscopic methods. The antifungal and antibacterial activity of the synthesized 1,3-oxazepine-dione derivatives were studied against Geotrichum sp., Escherichia coli, Klebsiella sp. and Staphylococcus aureus.
Experimental
Materials and Methods
Aldehydes, mines and other chemicals were purchased from Sigma Aldrich. Melting points were recorded on Electrothermal Melting point Apparatus (uncorrected). FT-IR spectra were recorded at room temperature from 4000-400cm-1 with KBr disc on Infrared Spectrophotometer Model Tensor 27 Bruker Co., Germany. The 1H-NMR spectra were recorded on Bruker Ac-300MHz spectrometer.
General Procedure for the synthesis of azomethine compounds R1-R4
Equimolar mixtures 0.01 mole of aromatic amines and 0.02 mole of aromatic aldehydes dissolved in 25 mL absolute ethanol was placed in a 100 mL round-bottom flask. 3-4 drops of glacial acetic acid was added as catalyst, the mixture was allowed to react at reflux temperature for 4 hours, and then let to cool down to the room temperature, the progress of the reaction and the purity of the compounds were monitored with TLC technique, whereby a crystalline solid was separated out and recrystallized from ethanol, the structural formula, names, melting points, colors and percentage of yields for the synthesized azomethine compounds R1-R4 are recorded [8-12].
General Procedure for the synthesis of 1,3-oxazepine-dione derivatives R5-R9
Equimolar mixtures 0.01 mole of azomethine compounds and 0.02 mole anhydride compounds dissolved in 30 mL of tetra hydro furan (THF) placed in a 100 mL round-bottom flask, the reaction mixture was refluxed for 3 hours, the progress of the reaction and the purity of the compounds were monitored with TLC technique then solid product was precipitated, filtered off and recrystallized from ethanol, the structural formula, names, melting points, colors and percentage of yields for the synthesized 1,3-oxazepine-dione derivatives R5-R9 were recorded [13, 14].
Anti-fungal and anti-bacterial activity of synthesized 1,3-oxazepine-dione derivatives R5-R
Anti-microbial activity of the derivatives against Geotrichum sp., E. Coli, Staphylococcus aureus and Klebsiella sp. Using well diffusion method on Mueller Hinton Agar plates. The well diameter is 6 mm. The zone of inhibition was recorded as antimicrobial activity. About 6 µg well-1 of chemicals in DMSO introduced into the bore wells on the agar using a sterile dropping pipette. The extracts were allowed to diffuse before inoculated with the yeast then incubated at 37 oC for 24 hour. The plates were examined for measuring any inhibition zone [15]. About 50 µg Gentamycine per well was used as a positive control and 50 µl DMSO was used as a negative control
Results and discussion
Chemistry
Tables 1 and 2 exhibited structural formula, nomenclature, the percentage of yield, melting point and the color of all prepared compounds. The best yield of the synthesized azomethine was for compounds R1 86% and R3 85%, while the lower yield was for compound R2 78% and the best yield of the synthesized 1,3-oxazepine-dione derivatives was for R5 89% while the lower yield was for R7 73%. The higher melting point for azomethine compounds was for compound R3, the lower melting point was for compound R4, while the higher melting point of the synthesized 1,3-oxazepine-dione derivatives was for compound R9, the lower melting point was for compound R5. The different colors, melting points and the number with distance of spots in the TLC technique to the products compared with the raw material are initial evidence of interaction.
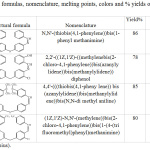 |
Table 1: Structural formulas, nomenclature, melting points, colors and % yields of azomethine compounds R1-R4. |
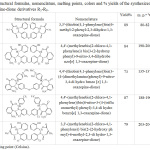 |
Table 2: Structural formulas, nomenclature, melting points, colors and % yields of the synthesized 1,3-oxazepine-dione derivatives R5-R9. |
Characterization of synthesized azomethine compounds R1-R4
Azomethine compounds were synthesized from commercially available aldehydes and primary amines. TLC technique was used to follow the chemical reaction, the synthesized azomethine identified by their melting points and FT-IR spectra. The FT-IR spectra showed the appearance of the stretching absorption bands of azomethine (C=N) at 1601-1628 cm-1 indicative of the formation of the resulting azomethine compounds, (C-N) at 1154-1167 cm-1, (C-S) at 687-692 cm-1, (C-Cl) at 933-937 cm-1 beside the characteristic bands of the residual groups in the structure [16].
The previous studies suggest that the aromatic aldehyde which contain such a -N(CH3)2 group at para position decreases the reaction speed when condensing with aromatic amine, while the reaction speed increases when such a -CF3 group in the same site on the aromatic aldehyde ring because the drawing electrons group such a -CF3 increase the the positive charge of the carbon of the carbonyle group (C=O) in aromatic aldehyde, while the donor electrons group such a -N(CH3)2 decrease the the positive charge of the carbon of the carbonyle group (C=O) in aromatic aldehyde, glacial acid is used as a catalyst to increase the electrophile of the carbonyl group (C=O) to accelerate the reaction and increase the precentage yield. See table 3 and FT-IR spectra of R3 and R4 compounds (figure 1).
Table 3: FT-IR of azomethine compounds R1-R4.
| FT-IRa, n(cm-1)b | |||||||
| Compound | C=N | C-N | C=C | C-H | C-H Aliphatic | Others | |
| Aromatic | Aromatic | Asymmetric | symmetric | ||||
| R1 | 1617 | 1166 | 1572 | 3028 | — | — | C-S: 692 |
| R2 | 1616 | 1154 | 1598 | 3052 | 2987 | 2937 | O-H: 3500 |
| R3 | 1601 | 1163 | 1574 | 3055 | 2977 | 2886 | C-S: 687 |
| R4 | 1628 | 1167 | 1578 | 3067 | 2989 | 2908 | C-F: 1307 |
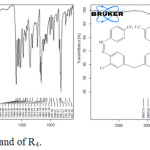 |
Figure 1: FT-IR spectra of R3 and of R4. |
The mechanism of azomethine compounds formation involves a nucleophile attack of the electron pair of NH2 amine on the C=O of aldehyde to form a hemiaminal N-substituted medium that loses a water molecule to give the stable compound (azomethine). The reaction is believed to occur in the following mechanism [17]. See (figure 2).
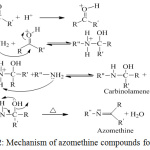 |
Figure 2: Mechanism of azomethine compounds formation. |
Characterization of synthesized 1,3-oxazepine-dione derivatives R5-R9
Synthesis of 1, 3- oxazepine- dione derivatives was achieved by the reaction of azomethine group (C=N) with anhydride. TLC techneque used to follow the chemical reaction, the resulted products were identified by their melting points, FT-IR and 1H-NMR spectra. The FT-IR spectra of the products showed disappearance of the absorption bands of the (C=N) of the azomethine compounds and the stretching absorption bands of two anhydride compounds and showed the appearance of the stretching absorption at 1608-1650cm-1 indicative of C=O lactam bonds, stretching absorption at 1698-1728cm-1 indicative of C=O lacton bonds, stretching absorption at 1277-1285cm-1 indicative of C-O bonds, stretching absorption at 1185-1203cm-1 indicative of C-N bonds, stretching absorption at 676-683 cm-1 indicative of C-S bonds, stretching absorption at (1345-1363)-(1482-1533)cm-1 indicative of NO2 group, beside the characteristic bands of the residual groups in the structure [18]. See table 4 and FT-IR spectra of R7 and R8 compounds (figure 3).
Table 4: FT-IR of the 1,3-oxazepine-dione derivatives R5-R9.
| FT-IRa (KBr), n(cm-1)b | |||||||||
| Compound | C=C | C-O | C-H | C-N | C=O | C=O | C-H Aliphatic Asymmetric Symmetric | Others | |
| Aromatic | Aromatic | Lactam | Lacton | ||||||
| R5 | 1586 | 1277 | 3102 | 1203 | 1624 | 1698 | 2975 | 2886 | C-S: 682 |
| R6 | 1559 | 1280 | 3049 | 1187 | 1608 | 1701 | 2985 | 2920 | O-H:3470 |
| R7 | 1599 | 1285 | 3098 | 1185 | 1650 | 1728 | 2934 | 2897 | C-S: 676 |
| R8 | 1573 | 1278 | 3042 | 1196 | 1629 | 1698 | 2939 | 2891 | C-F: 1307 |
| R9 | 1562 | 1282 | 3054 | 1186 | 1614 | 1715 | 2988 | 2929 | O-H:3480 |
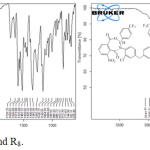 |
Figure 3: FT-IR spectra of R7 and R8. |
The 1H-NMR spectrum of R5 in solvent DMSO showed chemical shifts (δ ppm) as folllows: the singlet at 1.98 indicates the presence 6H of the two groups of (CH3), the singlet at 6.08 indicates the presence 2H of the two groups of (=CH), the singlet at 10.25 indicates the presence 2H of the two groups of (N-CH), multiplet at 7.24-7.95 indicates the presence 18H of the aromatic protons and the spectrum of R6 showed chemical shifts (δ ppm) as follows: the singlet at 4.04 indicates the presence 2H of the group (CH2), the singlet at 9.02 indicates the presence 2H of the two groups of (N-CH), the singlet at 13.16 indicates the presence 2H of two groups of (-OH), multiplet at 6.97-7.68 indicates the presence 20H of the aromatic protons [19]. Other chemical shifts (δ ppm) of compounds R7-R9 are given in (Table 5). See 1H-NMR spectra of R6 and R9 compounds (figure 4).
Table 5: The 1H-NMR Spectra of the 1,3-oxazepine-dione derivatives R5-R9 in DMSO.
| Compound | Chemical Shift δ ppma |
| R5 | Singlet in 1.98 for (6H, 2 CH3), singlet in 6.08 for (2H, 2 =CH), singlet in 10.25 for (2H, 2 N-CH), multiplet in 7.24-7.95 for (18H, aromatic protons). |
| R6 | Singlet in 4.04 for (2H, CH2), singlet in 9.02 (2H, 2 N-CH), singlet in 13.16 (2H, 2 -OH), multiplet in 6.97-7.68 (20H,aromatic protons). |
| R7 | Singlet in 3.03 for (12H, 4 N-CH3), singlet in 9.67 for (2H, 2 N-CH), multiplet and doublet of doublet in 6.78-8.44 for (22H, aromatic protons). |
| R8 | Singlet in 4.01 for (2H, CH2), singlet in 10.13 for (2H, 2 N-CH), multiplet and doublet of doublet in 6.69-8.71 for (20H, aromatic protons). |
| R9 | Singlet in 3.09 for (6H, 2 CH3), singlet in 4.04 for (2H, CH2), singlet in 5.20 for (2H, 2 =CH), singlet in 9.02 for (2H, 2 N-CH), singlet in 13.16 for (2H, 2 -OH), multiplet in 6.97-7.68 for (14H, aromatic protons). |
a The references point (the chemical shift of tetramethylsilane (CH3)4Si).
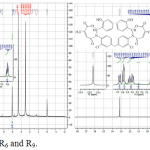 |
Figure 4: 1H-NMR Spectra of R6 and R9. |
The both reactions of the synthesized azomethine compounds with two anhydrides are given by the following equations (figure 5).
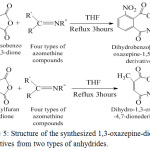 |
Figure 5: Structure of the synthesized 1,3-oxazepine-dione derivatives from two types of anhydrides. |
It may be concluded that both reactions take place via interaction between HOMO orbital of anhydrides with LUMO orbital of (C=N) group by concerted dipolar cycloaddition mechanism as represented in the following reaction [20], see figure 6.
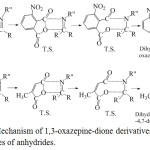 |
Figure 6: Mechanism of 1,3-oxazepine-dione derivatives formation for two types of anhydrides. |
The mechanism involves the addition of one σ-carbonyl to π-bond (C=N) to give 4- membered cyclic and 5-membered cyclic ring of anhydride in the same transition state [T.S.], which opens into 4-nitroisobenzofuran-1,3-dione and 3-methylfuran-2,5-dione anhydrides to give seventh-membered cyclic ring 1,3-oxazepine-dione derivatives [21].
The antifungal and antibacterial activity of 1,3-oxazepine-dione derivatives R5-R9
Table 6 exhibited that the higher zone of inhibition was 18.3 mm by compound R8 against S. aureus, followed 14.6 and 14.0 mm by the same compound against Klebsiella sp. and E. coli, respectively. The lower zone of inhibition was 8.0 mm by compound R9 against S. aureus and E. coli, and by compound R6 toward E. coli. While, compound R6 did not inhibit other microbes in this test. Also, the compounds R5 and R9 did not inhibit Geotrichum sp. and Klebsiella sp., respectively. The role of these compounds may be linked and destroyed the cell wall of microbes or stopped replication of microbial DNA [22, 23]. The differences in the inhibitory effect related to the chemical synthesis of each compound as above, see figures 7 and 8. Gentamycin showed the inhibition zone approx. 26 mg while the negative control (50 µl DMSO) did not exhibit any inhibition zone.
Table 6: Zone inhibition of the synthesized 1,3-oxazepine-dione derivatives R5-R9.
| Pathogenic fungi and bacteria | Zone inhibition (mm)a | Gentamycinb 50 µg/well | DMSOc 50 µg/well | ||||
| R5 | R6 | R7 | R8 | R9 | |||
| Geotrichum sp. | 0 | 0 | 11.1 | 12 | 0 | 26 | 0 |
| E. coli | 9 | 8 | 11.6 | 14 | 8 | 27 | 0 |
| S. aureus | 9 | 0 | 11.1 | 18.3 | 8 | 26 | 0 |
| Klebsiella sp. | 9 | 0 | 10 | 14.6 | 0 | 25 | 0 |
a Zone inhibition of the milimeter unit. b The positive control. c The negative control.
 |
Figure 7: Antimicrobial activity of the synthesized 1,3-oxazepine-dione derivatives R5-R9 in DMSO against Geotrichum sp. and E. Coli. |
 |
Figure 8: Antimicrobial activity of the 1,3-oxazepine-dione derivatives R5-R9 in DMSO against S. aureus and Klebsiella sp. |
Conclusions
The formation of stable seventh- membered heterocyclic derivatives as (dihydro-1,3-oxazepine-4,7-dione) and (dihydrobenzo [e] [1,3]oxazepine-1,5-dione) has been achieved by the interaction between HOMO orbital of anhydrides with LUMO orbital of (C=N). The results of FT-IR and 1H-NMR showed that the target molecules were formed due to the least obstructive effect in all preparation processes. TLC technique identified the synthesized compounds; this makes for all the synthesized compounds with high purity. Generally, R8 derivative is the best derivative that has significantly (p<0.01) recorded a stronger influence to inhibit the growth of all types of bacteria and fungi, while R6 derivative has recorded inhibition against one of the types of bacteria and fungi. Slight variation in the structure of those derivatives can show the very dramatic effect on the efficiency of these compounds in their bio-activity. The present work may be helpful in designing more potent antifungal and antibacterial agents for therapeutic use in the future.
Acknowledgments
Special thanks to Anbar University’s President Professor Dr. Khalid Battal Najim and Dean of the Applied Sciences – Heet College Professor Dr. Tahseen Ali Zaidan for their continuous support to publishing the research in certified international journals. Grateful is for Dr. Mustafa Nadhim Owaid, Al-Mustafa laboratory for helping to complete the bioactivity tests.
References
- El-ajaily, M.; Maihub, A.; Mahanta, U.; Badhei, G.; Mohapatra, R. P.; Das, mixed ligand complexes containing Schiff bases and their biological activities: a short review, Rasayan J. Chem. 2018, 11, 166-174.
- Nastasa, C.; Vodnar, D.; Ionu ¸ I.; Stana, A.; Benedec, D.; Tamaian, R.; Oniga, O.; Tiperciuc, B.; Antibacterial Evaluation and Virtual Screening of New Thiazolyl-Triazole Schiff Bases as Potential DNA-Gyrase Inhibitors, Int. J. Mol. Sci.2018, 19, 1-18.
- Bavane, J.; Mohod, R.; Synthesis, characterization and electrochemical studies of symmetrical schiff base complexes of [1-(5 chloro-2-hydroxy-4-methyl- phenyl) ethanone-4-chloro (-3-trifluro methyl) aniline], Pharma Innov. J.2018, 7, 149-152.
- Vadivel, R.; Jayakumar, R.; Ananthi, N.; Promising Antibacterial Activity of Simple Schiff Bases, Org. Med. Chem. Int. J. 2018,5, 1-6.
- Warad, I.; Abedalrazeq, H.; Amer, N.; Al-Nuri, M.; Al-Ali, A.; Al-Zaqri, N.; Shivalingegowda, N.; 1,3-Bis[(E)-(3-bromobenzylidene) amino] propan-2-ol, Molbank. 2017, 7,1-7.
- Mohammed-Ali, M.; Salman, H.; Abdul-Hussein, Z.; Synthesis, Characterization and Antibacterial Activity of Some New Oxazepine compounds, J. Thi-Qar Sci. 2014, 5, 32-37.
- Younus, A.; Jaber, N.; Synthesis and Characterization a New 1,3-Oxazepine Compounds from New Bis-4-Amino-3-mercapto-1,2,4-triazole Derivatives, Org. Chem.: Indian J. 2016, 12, 1-12.
- Iftikhar, B.; Javed, K.; Khan , M.; Akhter, Z.; Mirza, B.; Mckee, V.; Synthesis, characterization and biological assay of Salicylaldehyde Schiff base Cu (II) complexes and their precursors, J. Mol. Struct. 2018, 1155, 337-348.
- Jawoor P.G. Department of Studies in Chemistry, Karnatak University, Dharwad, IndiaS, S.; Patil P.G. Department of Studies in Chemistry, Karnatak University, Dharwad, IndiaCorrespondencepatil1956@rediffmail.com, S.; Toragalmath P.G. Department of Studies in, S.; Synthesis and characterization of heteroleptic Schiff base transition metal complexes: a study of anticancer, antimicrobial, DNA cleavage and anti-TB activity,S. J. Coord. Chem.1018, 71, 271-283.
- Kadhim, K.; Munahi, M.; Synthesis, Characterization and Biological Evaluation of Some Novel Schiff’S Bases Derived from Vanillin, J. Glob. Pharm. Technol. 2017, 10, 383-386.
- Azam, M.; Al-Resayes, S.; Wabaidur, S.; Altaf, M.; Chaurasia, B.; Alam, M.; Shukla, S.; Gaur, P.; Talmas, N.; Albaqami, M.; Islam, M.; Park, S.; Synthesis, Structural Characterization and Antimicrobial Activity of Cu (II) and Fe (III) Complexes Incorporating Azo-Azomethine Ligand, Molecules.2018, 23, 1-13.
- Al Zoubi, W.; Al‐Hamdani, A.; Ahmed, S.; Ko, Y.; a new azo‐Schiff base: Synthesis, characterization, biological activity and theoretical studies of its complexes, Appl Organometallic Chem. 2018,32, 1-15.
- Al-Sultani, K.; Synthesis, identification and evaluation tha biological activity for some new heterocyclic compounds derived from Schiff bases, IOSR J. Appl. Chem. 2017, 12, 39-47.
- H. Sadiq, World J. Pharm. Pharm. Sci., 2017, 6, 186-198.
- Owaid, M.; Raman, J. ; Lakshmanan, H.; Al-Saeedi, S.; Sabaratnam, V.; Al-Assaffii, I.; Mycosynthesis of silver nanoparticles from Pleurotus cornucopiae var. citrinopileatus and its inhibitory effects against Candida sp., Mater. Lett.2015, 153, 186-190.
- Silverstein, R.; Webster, F.; Kiemle D.; Spectrometric identification of organic compounds, John Wiley and sons, Inc., 7th edition, 2005, 72-126.
- Al-Juburi, R.; Synthesis and Characterization of Some Heterocyclic Compounds (Oxazepine, Tetrazole) Derived from Schiff Bases, Journal of Al-Nahrain University.2012, 15, 60-67.
- Altintop, M.; Çiftçi, G.; Temel, H.; Synthesis and evaluation of new benzoxazole derivatives as potential antiglioma agents, Marmara Pharm. J.2018, 22, 547-558.
- Silverstein, R.; Webster, F.; Kiemle, D.; Spectrometric identification of organic compounds, John Wiley and sons, Inc., 7thedition.2005, 127-202.
- Al- Bayati, R.; Al-Amiery, A.; Al- Majedy, Y.; Design, Synthesis and Bioassay of Novel Coumarins, Afr. J. Pure Appl. Chem.2010, 4, 74-86.
- Samir, A.; Rumez, R.; Fadhil, H.; Synthesis and characterization of some New Oxazepine Compounds Containing 1,3,4-Thiadiazole Ring Derived from D- Erythroascorbic Acid, Int. J. Appl. Chem.2017, 13, 393-407.
- McDonnell, G.; Russell, A.; Antiseptics and Disinfectants: Activity, Action, and Resistance, Clinic.l Microbio. Rev.1999, 12,147-179.
- Owaid, M.; Muslim, R.; Hamad, H.; Mycosynthesis of Silver Nanoparticles using Terminia sp. Desert Truffle, Pezizaceae, and their Antibacterial Activity, JJBS.1018, 11, 401-405.

This work is licensed under a Creative Commons Attribution 4.0 International License.









