Synthesis and Antimicrobial Activity of New Derivatives of Angular Polycyclic Phenoxazine Ring System
Mercy Amarachukwu Ezeokonkwo*1 , Kingsley Chizoba Iloka1, Uchechukwu Chris Okoro1, Efeturi Abreham Onoabedje1, Benjamin Ebere Ezema1, Fidelia Ngozi Ibeanu2, David Izuchukwu Ugwu1, Florence Uchenna Eze1 and Chiamaka Peace Uzoewulu1
, Kingsley Chizoba Iloka1, Uchechukwu Chris Okoro1, Efeturi Abreham Onoabedje1, Benjamin Ebere Ezema1, Fidelia Ngozi Ibeanu2, David Izuchukwu Ugwu1, Florence Uchenna Eze1 and Chiamaka Peace Uzoewulu1
1Department of Pure and Industrial Chemistry, University of Nigeria, Nsukka, 410001, Enugu State, Nigeria.
2Natural Science Unit, School of General Studies, University of Nigeria, Nsukka, 410001, Enugu State, Nigeria.
Corresponding Author E-mail: mercy.ezeokonkwo@unn.edu.ng
DOI : http://dx.doi.org/10.13005/ojc/350410
Article Received on : 08-06-2019
Article Accepted on : 05-07-2019
Article Published : 16 Aug 2019
Synthesis of angular polycyclic phenoxazine derivatives incorporating different phenols is reported in 30-99% yields. O-arylation of 6-chlorodibenzo[a,j] phenoxazin-5-one with a variety of electron-deficient, electron-neutral and electron-rich phenols under the catalytic palladium (II) acetate/t-BuXphos system furnished the compounds of interest. The highest yields were obtained when the intermediate was coupled with electron-rich phenols. IR, 1H-NMR, 13C-NMR and Mass spectra data, confirmed the structures of all the synthesized compounds. Study on the in vitro biological evaluation of the compounds against microorganisms revealed that they are potent antibacterial and antifungal agents, as they showed significant biological activity against Staphylococcus aureus, Streptococcus pneumonia, Escherichia coli, Salmonella typhi, klebsiella pneumonia, Pseudomonas aeruginasa, Basillus substilis, Candida albicans and Aspergillus niger.
KEYWORDS:Phenoxazines; Phenols; O-arylation; t-BuXphos; Antibacterial; Antifungal
Download this article as:| Copy the following to cite this article: Ezeokonkwo M. A, Iloka K. C, Okoro U. C, Onoabedje E. A, Ezema B. E, Ibeanu F. N, Ugwu D. I, Eze F. U, Uzoewulu C. P. Synthesis and Antimicrobial Activity of New Derivatives of Angular Polycyclic Phenoxazine Ring System. Orient J Chem 2019;35(4). |
| Copy the following to cite this URL: Ezeokonkwo M. A, Iloka K. C, Okoro U. C, Onoabedje E. A, Ezema B. E, Ibeanu F. N, Ugwu D. I, Eze F. U, Uzoewulu C. P. Synthesis and Antimicrobial Activity of New Derivatives of Angular Polycyclic Phenoxazine Ring System. Orient J Chem 2019;35(4). Available from: https://bit.ly/2MmUx74 |
Introduction
The continuous interest in functionalization of phenoxazine ring system has been attributed to the versatile and broad applications of the phenoxazines1 especially as dyes2 and drugs3. For example, Grzelakowska et al. synthesized several benzo[a]phenoxazine dyes containing molemide moiety4. Recently our group reported the synthesis of novel deeply coloured mixed phenothiazine-phenoxazine dyes1. Iweta et al. reported the synthesis of 2-amino-4,4α,7-dimethyl-3H-phenoxazine, which had antiproliferative, immunosuppressive, antibacterial, and antiviral effects5. Benzo[a]phenoxazine derivatives prepared by reacting vitamin-K3 and 2-aminophenol were found to possess significant cytotoxicity against MCF-76. Odin et al. also synthesized 1-amino-7-methyl-2,4,6,8,9-penta-azaphenoxazine found to possess good anti-inflammatory property7. Diazabenzo[a]phenoxazine derivatives containing sulphonamide derivatives synthesized by our group exhibited antibacterial and antifungal activities against the tested organisms8.
Phenoxazine ring system is a vital component of various drugs employed in medicine as anti-tumor, sedatives, antiepilectics, anti-inflammatory, antibacterial, tranquilizers, anti-tuberculosis, anti-depressant, and antioxidant9-11. They are also used industrially as acid-base indicators, laser dyes, biological strains, and chromophoric compounds12. In recent development, phenoxazines have been found very useful in the material sciences in form of organic light-emitting diodes13,14, thin film transistors15 and electrochromatic materials16. Their utility in these areas are attributed to their ability to undergo reversible oxidative reaction that leads to characteristic absorptions17. The extended conjugation observed in polycyclic phenoxazine, which often shows intense luminescience upon Uv-visible excitation has led to their utility as electrophore probes in supramolecules1. Apart from afore mentioned, phenoxazines are important component of some natural products such as ommatin D, actinomycin, questiomycin and cinnabarins. Questiomycin and actinomycin antibiotics have notable anti tuberculosis and anti-tumor activities respectively12. Recently, Wang and his coworkers reported the isolation of actinomycins A from marine Streptomyces, which exhibited cytotoxic and antibacterial activities against human cancer cell lines and Staphylococcus aureus and Enterococci strains respectively18.
Despite the several literatures on linear and angular phenoxazine derivatives, the chemistry of angular polycyclic phenoxazine ring has remained grossly underexplored. In continuation of our interest in phenoxazine chemistry, we report a facile synthesis of new angular polycyclic phenoxazine compounds containing phenoxazine-phenol conjugate.
Experimental
General
The reagents used in this work were of analytical grade. No further purification was done before use. The synthesized compounds were characterized at Department of Chemistry, School of Science and Engineering, University of Wakato, Hamilton, New Zealand. Determinations of melting points were done with the Gallenkamp melting point apparatus, Model No. 8899339 and they were uncorrected. Ultraviolet-visible spectra determination was done in ethanol, using the Shimadzu UV-1800 spectrometer. The absorption maxima are given in nanometers (nm) with log Є in parenthesis. Shimadzu 8400s Fourier Transform Infrared (FT-IR) spectrophotometer was used to determine the infrared spectra. The absorption is specified in wave number (cm-1). The 1H NMR and 13C NMR spectra determinations were carried out in CDCl3 via Bruker Avance DRX400 MHz spectrometer. The chemical shifts were recorded in δ-values (ppm) relative to tetramethylsilane. The mass spectra data were obtained in CH3OH, using high resolution Bruker Daltonic with electron spray ionization mode and positive ion polarity.
Synthesis of 6-chlorodibenzo[a,j]phenoxazin-5-one 3
1-Amino-2-naphtholhydrochloride 1 (7.2 g, 37 mmol) was dissolved in a mixture of DMF/Benzene (100 ml) contained in a three-necked flask. Anhydrous sodium carbonate (3.92 g) was added to the mixture and heated to boiling temperature. 2,3-Dichloro-1,4-napthoquinone 2 (8.4 g, 37 mol) was then added to the mixture and refluxed on a water bath with magnetic stirring for 6 hours at a temperature of 80°C. On cooling, the mixture was poured on crushed ice. The precipitate formed was filtered and air dried. Pure sample was obtained by recrystallization of the crude product from ethanol-water-mixture (2:1) to give dark brown powdery solid. The purity of the product was ascertained using thin layer chromatography. m.p 199-201°C (Lit. 200°C )19. Yield 4.5 g (56%), mp 199-200°C. UV-visible (ethanol), λmax: 234 (745), 257 (683), 272 (652), 327 (259), 408 (41). IR (Vmax, cm-1, KBR): 1681(C=O), 1587 (C=N), 1560 (C-C), 1455 (C=C), 1085 (C-O-C), 993 (C-H), 708 (C-Cl). 1H NMR (400 MHz, CDCl3): 7.7751-7.8681 (m, 4H), 7.9317-7.9766 (m, 4H), 8.0969-8.1203 (d, 2H). 13C NMR (100 MHz CDCl3): 176.4 (C=O), 142.9, 135.1-131.4, 127.5, 40.2, 39.9.
General procedure for synthesis of ploycycic phenoxazine derivatives 5a – i
An oven dried three necked flask fitted with a rubber septum was cooled to room temperature under a nitrogen purge. After removing the septum, palladium acetate (4.5 mg, 0.02 mmol, 2.0 mol%), ligand (0.03 mmol, 3.0 mol %), potassium phosphate (424 mg, 2,0 mmol), the phenol (1.2 mmol) and phenoxazine compound 3 (1.0 mmol) were introduced into the flask. The septum was replaced and toluene was poured into the flask through the septum. The flask was made air-tight with Teflon screw cap, and the mixture stirred at 100 – 110°C for 8 – 10 h. The progress of the reaction was monitor by thin layer chromatography. At the end of the reaction time, the mixture was left to cool. Ethyl acetate (40 ml) was then added to the mixture, after which it was filtered and concentrated. The crude product was recrystallized from hot ethanol-water (2:1) mixture to obtain the pure sample. Compounds 5a-i were prepared following the general procedure.
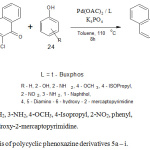 |
Scheme 1: Synthesis of polycyclic phenoxazine derivatives 5a – i. |
Synthesis of 6-(2-Aminophenoxyl)dibenzo[a,j]phenoxazin-5-one, 5a
The reaction of 2-aminophenol (0.131g, 1.2mmol) and 6-chlorodibenzo [a,j] phenoxazin-5-one (0.322g, 1.0mmol) gave a dark brown solid. Yield 970mg (37%), mp > 360°C. UV-Visible (ethanol) λmax: 234 (998), 254 (886), 280 (783), 389 (548), 437 (599), 647 (23). IR (Vmax, cm-1, KBr) 3376 and 3306 (NH2), 1677 (C=O), 1595 (C=N), 1563 (C-C), 1467 (C=C), 1384 (C-N), 1268 (Ar1-O-Ar2), 1074 (C-O-C), 987 (C-H), 1H NMR (400 MHz CDCl3): δ 3.70 (S, 2H), 6.44-6.50 (d, 2H), 6.64-6.78 (d, 2H), 7.34-7.60 (m, 4H), 7.77-7.92 (d, 4H), 8.40-8.42 (d, 1H), 8.78-8.80 (d, 1H). 13C NMR (100 MHz CDCl3): δ 134.5, 131.9-132.5, 124.9, 115.2-121.5, 29.7. HRMS (ESI), m/z (% relative intensity): 405.3792 [(1.56) M+ + 1], Calculated 404.425.
Synthesis of 6-(4-isopropylphenoxyl)dibenzo[a,j]phenoxazin-5-one, 5b
The reaction of 4-isopropylphenol (0.161 g, 1.2 mmol) and 6-chlorodibenzo[a,j] phenoxazin-5-one (0.322 g, 1.0 mmol) gave a black solid. Yield 830 mg (30%), mp > 360°C. UV-visible (ethanol) λmax: 228 (479), 240 (503), 264 (441), 331 (43), 414 (6). IR (Vmax, cm-1, KBr): 1675 (C=O), 1593 (C=N), 1529 (C-C), 1460 (C=C), 1270 (Ar1-O-Ar2), 1071 (C-O-C), 991 (C-H). 1H NMR (400 MHz CDCl3): δ 0.89-0.9 (d, 6H), 2.83-2.88 (S, 1H), 7.55-7.78 (m, 4H), 7.06-7.20 (m, 4H), 7.55-7.78 (m, 4H), 8.14-8.18 (d, 2H). 13C NMR (100 MHz CDCl3): δ 134.2, 126.7, 116.3, 22.6-24.2 (C-H), 31.9-33.3 (CH3 of isopropyl). HRMS (ESI), m/z ( HH% relative intensity): 429.3667 [(100) M+ -2], Calculated 431.491.
Synthesis of 6-phenoxyldibenzo[a,j]phenoxazin-5-one, 5c
The reaction of phenol (0.113 g, 1.2 mmol) and 6-chlorodibenzo[a,j]phenoxazin-5-one (0.322 g, 1.0 mmol) gave a dark brown solid. Yield 1540mg (60%), mp > 360°C. UV-Visible (ethanol) λmax: 228 (947), 261 (778), 326 (197), 474 (142), 647 (20). IR (Vmax, cm-1, KBr): 1676 (C=O), 1592 (C=N), 1489 (C-C), 1271 (Ar1-O-Ar2), 1074 (C-O-C), 992 (C-H). 1H NMR (400 MHz CDCl3): δ 6.9111-6.9562 (t,3H), 6.8332-6.8530 (d,2H), 7.015-7.3465 (m, 4H), 7.5433-7.8212 (m, 4H), 8.1093-8.1209 (d, 1H), 8.2344-8.25246 (d, 1H), 6.9111-6.9562 (t,3H), 6.8332-6.8530 (d,2H), 7.015-7.3465 (m, 4H), 7.5433-7.8212 (m, 4H), 8.1093-8.1209 (d, 1H), 8.2344-8.25246 (d, 1H). 13C NMR (100 MHz CDCl3): δ 134.3, 126.9-129.4, 22.7-31.9. HRMS (ESI), m/z ( HH% relative intensity): 391.66 [(1.04) M+ +2], Calculated 389.41.
Synthesis of 6-(4-methoxyphenoxyl)dibenzo[a,j]phenoxazin-5-one, 5d
The reaction of 4-methoxyphenol (0.148 g, 1.2 mmol) and 6-chlorodibenzo[a,j] phenoxazin-5-one (0.332 g, 1.0 mmol) gave a black solid. Yield 2000 mg (74%), mp > 360°C. UV-visible (ethanol) λmax: 230 (667), 264 (604), 328 (160), 391 (83), 471 (92), 645 (13). IR (Vmax, cm-1, KBr): 1675 (C=O), 1592 (C=N), 1531 (C-C), 1459 (Ar1-O-CH3), 1271 (Ar1-O-Ar2), 1073 (C-O-C), 991 (C-H). 1H NMR (400 MHz CDCl3): δ 3.80 (S, 3H), 6.80-7.23 (m, 4H), 7.54-7.77 (m, 4H), 7.83-7.90 (m, 4H), 8.19-8.26 (d, 2H). 13C NMR (100 MHx CDCl3): δ 114.8-116.0, 22.7-31.9 (C-CH3). HRMS (ESI), m/z ( HH% relative intensity): 420.43 [(33.12) M+ + 1], Calculated 419.436.
Synthesis of 6-(4,5-diamino-6-hydroxyl-2-mercaptopyrimidine)dibenzo[a,j] phenoxazin-5-one, 5e
The reaction of 4, 5-diamino-6-hydroxyl-2-mercaptopyrimidine (0.181 g, 1.2 mmol). and 6-chlorodibenzo[a,j]phenoxazin-5-one (0.332 g, 1.0 mmol) gave a brown solid. Yield 2900 mg (99%), mp > 360°C. UV-Visible (ethanol) λmax: 220 (159), 274 (151), 306 (118). IR (Vmax, cm-1, KBr): 3402 and 3311 (2NH2), 1655 (C=O), 1579 (C=N, C-C), 1458 (C=C), 1378 (C-N), 1207 (Ar1-O-Ar2) 1071 (C-O-C), 990 (C-H), 623 (C-S). 1H NMR (400, MHz CDCl3): δ 5.98 (S, 4H), 7.27 (m, 4H), 8.18 (d, 2H). 13C NMR (100 MHz CDCl3): δ 172.8 (C=O), 163.2, 107.6, 34.2. HRMS (ESI), m/z (% relative intensity): 456.14 [(1.83), M+ + 1], Calculate455.498.
Synthesis of 6-(2-nitrophenoxyl)dibenzo[a,j]phenoxazin-5-one, 5f
The reaction of 2-nitrophenol (0.166 g, 1.2 mmol) and 6-chlorodibenzo[a,j]phenoxazin-5-one [0.332 g, 1.0 mmol) gave a dark brown solid. Yield 1760 mg (63%), mp > 360°C. UV-Visible (ethanol), λmax: 206 (668), 226 (74.9), 264 (643), 332 (130), 422 (37), 473 (26). IR (Vmax, cm-1, KBr): 1675 (C=O), 1593 (C-NO2), 1444 (C=C), 1271 (Ar1-O-Ar2), 1069 (C-O-C), 989 (C-H). 1H NMR (400 MHz CDCl3): δ 6.10 – 7.20 (m, 4H), 7.23-7.77 (m, 4H), 7.81-8.04 (m, 4H), 8.19-8.23 (d, 3H). 13C NMR (100 MHz CDCl3): δ 22.69-31.92. HRMS (ESI), m/z (% relative intensity): 436.23 [(1.59) M+ + 1], Calculated 434.407.
Synthesis of 6-(3-aminophenoxyl)dibenzo[a,j]phenoxazin-5-one, 5g
The reaction of 3-aminophenol (0.130 g, 1.2 mmol) and 6-chlorodibenzo[a,j] phenoxazin-5-one (0.332 g, 1.0 mmol) gave a dark brown solid. Yield 2700 mg (98%), mp > 360°C. UV-visible (ethanol) λmax: 230 (946), 257 (894), 283 (822), 390 (177), 475 (301), 509 (8), IR (Vmax, cm-1, Kbr): 3372 and 3253 (NH2), 1677 (C=O), 1596 (C=N), 1565 (C-C), 1446 (C=C), 1339 (C-N), 1295 (Ar1-O-Ar2), 1067 (C-O-C), 992 (C-H). 1H NMR (400 MHz CDCl3): 3.72 (S, 2H), 6.73 (d, 2H), 6.59 (d, 1H), 7.10 (S,1H), 7.21-7.25 (m. 4H), 7.54-7.82 (m, 4H), 8.14 d, 1H), 8.22 (d, 1H). 13CNMR (100 MHz CDCl3): δ 22.69-31.91. HRMS (ESI), m/z (% relative intensity): 404.33 [2.10) M+], Calculated 404.425.
Synthesis of 6-(1-naphthoxyl)dibenzo[a,j]phenoxazin-5-one, 5h
The reaction of 1-naphthol (0.173 g, 1.2 mmol) and 6-chlorodibenzo[a,j]phenoxazin-5-one (0.332 g, 1.0 mmol) gave a dark brown solid. Yield 2100 mg (74%), mp > 360°C. UV-Visible (Ethanol) λmax: 200, 227 (283), 277 (865), 359 (162), 471 (119). IR (Vmax, cm-1, KBr); 1666 (C=O), 1592 (C=N), 1519 (C-C), 1459 (C=C), 1278 (Ar1-O-Ar2), 1067 (C-O-C), 992 (C-H). 1H NMR (400 MHz CDCl3): δ 6.39-6.42 (d, 1H), 7.45-7.51 (m, 4H), 7.72- 7.81 (m, 4H), 8.40 (d, 1H), 8.50 (d, 1H). 13C NMR (100 MHz CDCl3): δ 135.26, 122.17-127.93, 87.03. HRMS (ESI), m/z (% relative intensity): 441.10 [(7.10) M+ + 2], Calculated 439.47.
Synthesis of 6-(4-chlorophenoxyl)dibenzo[a,j]phenoxazin-5-one, 5i
The reaction of 4-chlorophenol (0.154 g, 1.2 mmol) and 6-chlorodibenzo[a,j] phenoxazin-5-one (0.332 g, 1.0 mmol) gave a black solid. Yield 1320 mg (48%), mp > 360°C. UV-Visible (ethanol) λmax: 200, 222 (825), 239 (719), 263 (587), 326 (90), 414 (36), 472 (34). IR (Vmax, cm-1 KBr); 1672 (C=O), 1444 (C=C), 1269 (Ar1-O-Ar2), 1075 (C-O-C), 989 (C-H), C-Cl (617). 1H NMR (400 MHz CDCl3): δ 7.15-8.03 (d, 2H). 13C NMR (100 MHz CDCl3): δ 40.15, 39.95-39.73. HRMS (ESI), m/z (% relative intensity): 423.53 [(29.10) M+], Calculated 423.855.
Antimicrobial activity study
The antimicrobial activity of the compounds was evaluated in form of minimum inhibitory concentration (MIC) against the tested microbes via micro-broth dilution method following the procedure stipulated by Clinical Laboratory Standard Institute20. The microorganisms in this study are Staphylococcus aureus, Streptococcus pneumonia, Escheria coli, Salmonella typhi, klebsiella pneumonia, Pseudomonas aeruginosa, Bacillus subtilis, Candida albicans and Aspergillus nigger. These are clinical isolates obtained from the Department of Pharmaceutical Microbiology and Biotechnology Laboratory, University of Nigeria, Nsukka. The standard antibiotics used were ciprofloxacin and fluconazole. The 500 µg/ml stock solution of the compounds was prepared by dissolving 10 mg of each compound in 20 ml of 50% DMSO. Different concentrations (100µg/ml, 80 µg/ml, 50 µg/ml, 40 µg/ml, 30µg/ml, 20µg/ml and 10µg/ml), were obtained through serial dilution of the stock solution20. The molten agar plates with different concentrations of the compounds were allowed to gel. The plates were divided into nine equal parts with permanent marker. The test organisms were standardized using 0.5 Mac Faland turbid equivalent. They were streaked on the segments, and labelled. The culture plates were incubated in inverted position at 37°C for 24 h, and at 25°C for 48 h for fungi. After the due period of incubation, the plates were observed for sensitivity and resistivity of the organisms to the compounds, and further incubated for another 24 h at 37°C, and 48 h at 25°C to determine whether the activity was bactriostatic or bactriocidal. The observation was also recorded. The same procedure was carried out for the reference antibiotic drugs (ciprofloxacin and fluconazole).
Results and Discussion
Chemistry
The synthesis of the novel polycyclic phenoxazine derivatives (5a-I) was accomplished by catalyst assisted cross-coupling reaction between 6-chlorodibenzo[a,j]phenoxazin-5-one (3) and various phenols (4) (Scheme 1). The intermediate 3 was earlier prepared by refluxing equimolar mixture of 1-amino-2-naphtholhydrochloride and 2,3-dichloro-1,4-napthoquinone at 80°C in the presence of anhydrous sodium carbonate. The dark brown crystalline solid was obtained after work-up and subsequent recrystallization from ethanol-water mixture. The cross-coupling reaction between compound 3 and electron-rich, electron-deficient and electron-neutral phenols, catalyzed by catalyst system consisting of palladium (II) acetate and t-BuXphos furnished the new polycyclic phenoxazine derivatives (5a – i) in 30-99% yields. The polycyclic phenoxazine derivatives were obtained in higher yields when the intermediate (3) coupled with electron rich phenols (4d, 4e and 4g). 4,5-Diamino-6-hydroxy-2-mercapto pyridine (4e) gave the highest yield (99%) due to presence of increase electron-donating groups. Compound 4a gave a lower yield than 4g even though they both contain electron-donating amino group. The low yield in compound 4a is probably due to steric effect. The effect of increase in conjugation is seen in the case of reaction of compound 3 with 1- naphthol (5h), which gave a better yield (74%) than phenol (60%).
On the other hand a low yield was obtained when compound 3 reacted with electron-deficient phenol 4i (48%). This is consistent with those earlier reported in literature21,22. The observation may be due to the electron-deficient nature of the phenol (4i).
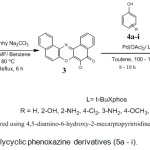 |
Scheme 2: Synthesis of polycyclic phenoxazine derivatives (5a – i). |
Spectra characterization
The UV-visible spectra of compounds 5a-i showed a bathochromic shift in absorption maxima from 408 nm in compound 3 to 414 -509 nm, which is due to the extended pi-conjugation system in the molecules. The IR spectra of compounds 5a-i showed the characteristic C-O-C asymmetric stretching band of diaryl ethers at 1268-1295 cm-1. Additional band was observed in the spectrum of compound 5d at 1072 cm-1 due to C-O stretching of CH3-O. The peaks at 1665-1677 cm-1 observed in all the spectra correspond to the absorption band of carbonyl group (C=O), which forms O-hydroxyl aryl intramolecular hydrogen bonding as shown in Scheme 2. Compound 5a showed the expected asymmetric and symmetric stretching vibrations of the two NH bonds of aromatic primary amines at 3376 and 3306 cm-1 respectively. Similar peaks were observed for compounds 5e and 5g at 3402 and 3311 cm-1, and 3372 and 3253 cm-1 respectively due to the NH vibrations. Compound 5f showed two characteristic bands at 1523 and 1330 cm-1 attributed to asymmetric and symmetric stretching vibrations of aromatic NO2 group. The weak band around 2580 cm-1 in the IR spectrum of compound 5e was attributed to the S-H stretching of thiols. The C-H stretching of CH3 was observed at 2980 cm-1 in the spectrum of compound 5d. The bands at 1369 and 1325 cm-1 in the spectrum of compound 5b were attributed to C-H stretching of C-CH3 group of isopropyl in the compound.
The assigned structures for the synthesized compounds were further supported by the NMR spectra of the compounds. The aromatic protons showed multiplet peaks at range of δ 7.00-8.03 ppm. Compound 5a showed a singlet peak at 3.70 equivalent to 2H. This peak was attributed to NH2 protons. Similar peaks were seen at 5.98 and 3.72 in the spectra of compounds 5e and 5g respectively due to NH2 protons. A doublet at 0.89-0.91 equivalent to 6H of two methyl group was observed in the spectrum of compound 5b. The protons of methoxy group in compound 5d were observed as singlet at 3.80.
The mass spectra data of compounds 5a-i can be seen in the experimental section. The molecular ions are in agreement with the proposed molecular formula of the compounds.
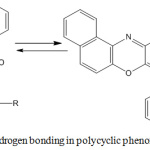 |
Scheme 3: Intramolecular hydrogen bonding in polycyclic phenoxazine derivatives 5a-i. |
Antimicrobial Activity
The compounds showed good and varying inhibition activity against the test microbes. The MICs of the compounds are presented in Table 1. The MIC values of the compounds ranges from 20 to 100 μg/mL while those of ciprofloxacin and Fluconazole was 30µg/ml. Although ciprofloxacin and fluconazole were more active than most of the synthesized compounds, it was apparent that some of the compounds have MIC (30 µg/ml) comparable to those of ciprofloxacin. It is also noteworthy that compounds 5e and 5g (MIC = 20 µg/ml) showed better activity than ciprofloxacin (MIC = 30µg/ml) against Bacillus subtilis and Escheria coli respectively. Pseudomonas aeruginosa was resistant against most of the compounds except compounds 5h and 5i. Streptococcus pneumonia was only affected by compounds 5c, 5d, and 5i. All the compounds were active against Escheria coli, Bacillus subtilis and Candida albicans with MIC values ranging from 20 to 80 µg/ml. It was also observed that the compounds were bactriocidal. These compounds can actually serve as leads for antimicrobial drugs especially against bacterial strains.
Table 1: Minimum inhibition concentration (MIC) of the polycyclic phenoxazine derivatives (µg/ml).
| Microorganisms → ↓ Compounds | Staph. Aureus. | Strep. Pneu. | E. coli | Sal. typhi | Kleb pneu | Pseu. Aeru. | B. subtilis | C. albs | As. niger |
| 5a | 50 | + | 40 | 80 | + | + | 30 | 80 | + |
| 5b | 80 | + | 80 | + | + | + | 50 | 80 | + |
| 5c | 30 | 80 | 40 | 50 | 50 | + | 40 | 50 | 80 |
| 5d | 30 | 50 | 40 | 50 | 40 | + | 30 | 40 | 80 |
| 5e | + | + | 80 | 100 | + | + | 20 | 80 | + |
| 5f | 50 | + | 80 | 30 | 50 | + | 80 | 80 | + |
| 5g | 80 | + | 20 | 30 | + | + | 50 | 50 | 80 |
| 5h | 50 | + | 40 | 80 | 100 | 40 | 40 | 40 | 40 |
| 5i | 40 | 80 | 30 | 30 | 80 | 80 | 30 | 50 | 80 |
| Ciprofloxacin 30µg/ml | – | – | – | – | – | – | – | + | + |
| Fluconazole 30µg/ml | + | + | + | + | + | + | + | – | – |
Conclusion
Series of new angular polycyclic phenoxazines containing electron-rich/electron-deficient/electron-neutral phenols have been synthesized via palladium (II) acetate/t-BuXphos assisted cross-coupling reaction. The coupling transformation occurred smoothly in non-polar solvent giving good to excellent yields. The polycyclic phenoxazine derivatives were found to have significant biological activities against Staphylococcus aureus, Streptococcus pneumoni, Escheria coli, Salmonella typhi, klebsiella pneumonia, Pseudomonas aeruginosa, Bacillus subtilis,Candida albicans and Aspergillus nigger. Some of the compounds have activity comparable to that of standard Ciprofloxacin drug. Hence they can serve as potential lead for antibacterial drugs.
Acknowledgements
This work was supported by TETFUD Nigeria under the TETFUND Institution Based Research Grant (TETFUND/DESS/UNI/NSUKKA/RP/VOL.V, 2016). The Organic/Medicinal Chemistry Research group is sincerely grateful to TETFUND for this support.
Conflict of Interest
There is no conflict of interest.
References
- Onoabedje, E.A.; Chinwuko, O.C.; Ezema, B.E.; Ezeokonkwo, M. A. Phosphorus, Sulfur, and Silicon and the Related Elements. 2018, 1563-5325. DOI:10.1080/10426507.2018.1436545
- Mass, H.; Khatr, A.; Calzaferri, G.; Microporous material,2003, 65, 233-224.
- Odaa M. E.; Okoro, U. C; Ugwu, D. I. Asian J. Chem.2015, 28(7)3069-3073.
- Grzelakowska, A., Kolinsha, I., Zakdos – szyda, M.; Michalski, R.; Sokolowska, J. Coloration Technology, 2016, 1333(2), (2016), 145 – 157. dol; 10.1111/cote.12261.
- Iweta, A.; Yamaguchi, T.; Sato, K.; Izumi, R.; Tomoda, A. Tohoku J. Exp Med., 2003, 200, 161-165.
- Chadar, D.; Rao, S.S.; Khan, A.; Geji, S.P.; Bhat, S.S.; Weyhermuller, T.; Salunke – Gawali, S. RSC Advance, 2015, 5(71), 57929. doi; 1039/C5RA084996B.
- Odin, E.M.; Onojah, P.K.; Akpanisi, L.E.; Akabueze, B.O. American Chemical Science Journal, 2016, 16(4), 1- 2. dol;10.9734/AC51/2016/25008.
- Ezeokonkwo, M.A.; Eze, C.C.; Okafor, S.N.; Onoabedje, E.A.; Godwin – Nwakwasi, E.U.;Ibeanu, F.N. Medical chemistry Research, 2018, ISSN 1054 – 2523. dol;10.1007/5000441 – 018 – 2251 – 4.
- Kapur, S.; Clelland, R.M. U.S patient, 6890919, (2005).
- Onoabedje, E. A.; Okoro, U. C.; Sarka, A.; Knight, D. W. J. Heterocycl. Chem.2016, 53,1787-1794. Doi: 10.1002fjhet-2485.
- Okoro, U.C.; Ezeokonkwo, M.A.; Utime, A.; Godwin-Nwakwasi, E.U.; Ibeanu, F.N.; Okafor, S.N. Asian J. Chem., 2018, 30 (10), 2317-2321.https://doi.org/10.14233/ajchem.2018.21477.
- Okoro, U. C.; Ezeokonkwo, M. A.; Ujah, E.A.; Nweloke, R.N. Asian J. Chem., 2015, 28(2), 317-324.
- Park, Y. ; Kim, B.; Lee, C.; Hyun, A. ; Jang, S.; Lee, J.H.; Gal, Y.S.; Kim, T.H.; Kim,K.S.; Park, J. Jor. Phy. Chem. 2015, X 115, 4843-4850. doi: 10.1021/p200902h.
- Onoabedje, E. A.; Egu, S. A.; Ezeokonkwo, M. A.; Okoro, U.C. J. molecular structure,2018, 1175, 956-962.
- Kulkania, A.P.; Zhu, Y.; Babel, A.; Wu, P.T.; Jenekbe, A. Chem. Mater, 2008, 20(13),4212-4223, doi: 10.1021/cm7022136.
- Zhu, Y.; Babel, A.; Jemelche, S.A. Macromolecules, 2005, 38, 7983-7991. doi:10.1021/ma0510993.
- Kramer, C.S.;Zeitler, K.; Muller, T.J. J. Tetrahedron Lett., 2001, 42,8619– 8620.doi:10.1016/S0040-4039(01)01848-2.
- Wang, Q; Zhang, Y.X.; Wang, M.; Tan, Y.; Hu, X.; He, H.; Xiao, C.; You, X.; Wang,Y.; Gan, M. Scientific Reports. 2017, 7, 3591. Doi: 10.1038/s41598-017-03769-8.
- Okafor C. O.Dyes and Pigments, 1986, 7(2), 103-131.doi.org/10.1016/0143- 7208(86)85003-3.
- Clinical Laboratory Standards Institute, Performance standards for Antimicrobial Disc Dilution Susceptibility Tests for Bacteria isolated from Animal. 2002, 22, 13-14.
- Mann, G.; Hartwig, J.F.; Tetrahedron let.1997, 038, 8005 – 8008.
- Hartwig, J.F. Acc. Chem. Res. 1998, 31, 852 – 860.

This work is licensed under a Creative Commons Attribution 4.0 International License.









