Preparation of Benzimidazole Based Coumarin Derivatives as Antimicrobial and Antioxidant Agents
Mohd. Imran*
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Northern Border University, Rafha-91911, Saudi Arabia.
Corresponding Author E-mail: imran_inderlok@yahoo.co.in
DOI : http://dx.doi.org/10.13005/ojc/350420
Article Received on : 01-07-2019
Article Accepted on : 17-08-2019
Article Published : 20 Aug 2019
The aim of the present work was to afford benzimidazole-based coumarins as antimicrobial and antioxidant agents. The compounds 3a-3j were prepared by reaction the compounds 1a-1b with the compounds 2a-2e in acetone. Chemical structures of 3a-3j were proven by their spectral analysis. Minimum inhibitory concentrations of 3a-3j were recorded by serial dilution procedure. Antioxidant potential was assessed by the 1,1-diphenyl-2-picrylhydrazyl method. Compounds 1a and 3g were identified as promising antimicrobial agents. Most of the compounds displayed moderate antioxidant activity. It has been concluded that the replacement of the 2-butylthio group with 2-pentylthio or 2-hexylthio substituents and the coumarin structure with another close flavonoid structure may provide better dual antimicrobial/antioxidant compounds.
KEYWORDS:Antimicrobial; Antioxidant; Benzimidazole-Based Coumarins; Synthesis
Download this article as:| Copy the following to cite this article: Imran M. Preparation of Benzimidazole Based Coumarin Derivatives As Antimicrobial and Antioxidant Agents. Orient J Chem 2019;35(4). |
| Copy the following to cite this URL: Imran M. Preparation of Benzimidazole Based Coumarin Derivatives As Antimicrobial and Antioxidant Agents. Orient J Chem 2019;35(4). Available from: http://www.orientjchem.org/?p=59205 |
Introduction
Antimicrobial resistance is a worldwide concern of the present time.1 This problem is further supported by the emergence of newer microbial diseases.2 Antioxidants are reported to limit the development of countless diseases like cardiovascular diseases, diabetes, inflammation, and cancer triggered by the high level of reactive oxygen species.3 It is also evident from the published reports that increased oxidative stress decreases the healing rate of infected tissue.4 Accordingly, it is worthy to develop new medicinal compounds possessing appreciable antioxidant activity for the management of microbial infections. Benzimidazoles and coumarins are reported as antibmicrobial5, antiviral6, antidiabetic7,8, antihypertensive9,10, antitumor11, and antioxidants12,13. In light of the literature, it looked worthful that a combination of benzimidazole moiety and coumarin moiety may provide medicinal compounds that possess appreciable antioxidant activity as well as antimicrobial potential. Therefore, an extension of our work associated to the antimicrobial agent1,2,14,15 and based on the existing literature, it was aimed to develop benzimidazole based coumarin derivatives with the expectation of providing improved antimicrobial compounds.
Materials and Methods
General
The uncorrected .m.p. (melting point) were obtained by means of the open-capillary tube method. IR (KBr, cm-1), NMR (δ in ppm, DMSO-d6), and Mass (m/z) analysis was performed using Nicolet, 5PC FT-IR spectrometer, Bruker DRX-300 FT NMR, and Jeol-JMS-D-300, respectively. The carbon, hydrogen, and nitrogen analysis remained in the range of ± 0.4% concerning the calculated values. The reaction monitoring and purity assessment were performed by TLC. The Rf value was measured by means of benzene:acetone (7:3).
Preparation of intermediates 1a-1b
The intermediates 1a-1b were prepared according to our prior publication16,17.
Preparation of intermediates 2a-2e
The intermediates 2a-2e were prepared according to our prior publication1.
Synthesis of 3-(2-(2-(butylthio)-1H-benzo[d]imidazol-1-yl)acetyl)-2H-chromen-2-one (3a)
A mixture of 1a (0.1 mol), 2a (0.1 mol), sodium carbonate (0.1 mol), and acetone (40 ml) was stirred for 18 H at 25°C. The subsequent mass was concentrated to 20 ml and it was decanted into the iced water. The resulting compacted bulk was filtered, and crystallized by EtOH.
Compounds 3b-3j were prepared in a similar manner. The synthetic pathway of the compounds 3a-3j is provided in Figure 1.
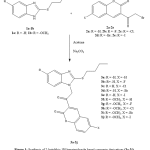 |
Figure 1: synthesis of-2butylthio-1H-benzimidazole based coumarin derivatives(3a-3j) Click here to view figure |
Antimicrobial Activity
The compounds 1a-1b and 3a-3j were evaluated for their antimicrobial action compared to S. aureus (ATCC-25923), E. coli (ATCC-25922), E. faecalis (ATCC-29212), K. pneumoniae (ATCC-700603), C. albicans (ATCC-2091), and P. citrinum (NCIM-768) as per our prior publications.1,2,14,15 Ofloxacin and ketoconazole having different concentrations were used as standard drugs. The compounds were also tested at different concentrations to determine their minimum inhibitory concentrations (MICs). The standard and test solutions were prepared in sterile dimethyl sulfoxide (DMSO). Sterile DMSO also functioned as control.
Anti-tubercular activity
The anti-tubercular activity was done against Mycobacterium intercellulari (ATCC 35734), and Mycobacterium smegmatis (ATCC 35797) as per our prior publication.18 In short, different concentrations of isoniazid, the compounds 1a-1b, and the 3a-3j were prepared in sterile dimethyl sulfoxide (DMSO) to determine their minimum inhibitory concentrations (MICs). The agar medium was used to carry out the anti-tubercular activity. Isoniazid was used as standard drug. The sterile DMSO was used as control.
Antioxidant activity
The DPPH procedure19,20 was used to perform the antioxidant activity. In brief, an ethanolic 0.1 mM solution of DPPH was prepared, which also served as control. Various concentrations of ascorbic acid and the test compounds were prepared in EtOH. Two ml of the control solution was mixed with the standard solution (6 ml) and the test compound solutions (6 ml). The subsequent mixture was shaken and stored at 25oC for ½ H. The absorbance of the test solution, standard solution, and the control was measured at 517 nm. The test was performed in triplicate.
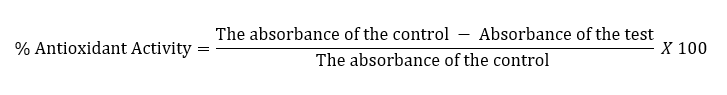
Results
The physical and spectral analysis data of 3a-3j are given below.
3-(2-(2-(butylthio)-1H-benzo[d]imidazol-1-yl)acetyl)-2H-chromen-2-one (3a)
Rf: 0.82; m.p.: 155-157°C; % yield: 60; IR: 1713 & 1701, C=O; 1620, C=N; 1555, C=C; 1225, C–O–C; 1H-NMR: 0.93 (t, 3H), 1.55-165 (m, 4H), 3.17 (t, 2H), 5.40 (s, 2H), 7.31-7.71 (m, 8H), 8.31 (s, 1H); 13C-NMR: 14.3, 22.5, 33.3, 37.3, 60.5, 111.0, 116.1, 117.1, 119.0, 124.0 (2C), 126.3, 128.8, 129.2, 132.1, 135.1, 138.3, 139.8, 153.7, 154.1, 159.9, 165.5; Mass: 392 (M+); Elemental Analysis, Found (Calcd.) for C22H20N2O3S: C, 67.25 (67.33); H, 5.18 (5.14); N, 7.15 (7.14).
3-(2-(2-(butylthio)-1H-benzo[d]imidazol-1-yl)acetyl)-6-fluoro-2H-chromen-2-one (3b)
Rf: 0.84; m.p.: 177-179°C; % yield: 50; IR: 1710 & 1695, C=O; 1620, C=N; 1550, C=C;, 1220, C–O–C; 1H-NMR: 0.93 (t, 3H), 1.56-164 (m, 4H), 3.21 (t, 2H), 5.44 (s, 2H), 7.30-7.70 (m, 7H), 8.31 (s, 1H); 13C-NMR: 14.3, 22.5, 33.3, 37.3, 59.9, 111.0, 115.5, 116.7, 117.0, 123.8 (2C), 124.8, 126.0, 132.1, 135.1, 138.5, 139.9, 149.5, 153.5, 159.1, 160.5, 165.3; Mass: 410 (M+); Elemental Analysis, Found (Calcd.) for C22H19FN2O3S: C, 64.30 (64.38); H, 4.66 (4.67); N, 6.84 (6.82).
3-(2-(2-(butylthio)-1H-benzo[d]imidazol-1-yl)acetyl)-6-chloro-2H-chromen-2-one (3c)
Rf: 0.75; m.p.: 149-151°C; % yield: 55; IR: 1721 & 1710, C=O; 1625, C=N; 1560, C=C; 1225, C–O–C; 1H-NMR: 0.96 (t, 3H), 1.55-165 (m, 4H), 3.19 (t, 2H), 5.43 (s, 2H), 7.31-7.71 (m, 7H), 8.36 (s, 1H); 13C-NMR: 14.2, 22.6, 33.4, 37.2, 59.3, 111.1, 116.2, 119.0, 124.0 (2C), 124.5, 127.8, 129.9, 132.1 (2C), 135.1, 138.4, 139.9, 150.0, 153.5, 160.3, 165.5; Mass: 426 (M+); Elemental Analysis, Found (Calcd.) for C22H19ClN2O3S: C, 61.87 (61.90); H, 4.45 (4.49); N, 6.55 (6.56).
6-bromo-3-(2-(2-(butylthio)-1H-benzo[d]imidazol-1-yl)acetyl)-2H-chromen-2-one (3d)
Rf: 0.76; m.p.: 140-142°C; % yield: 60; IR: 1710 & 1699, C=O; 1628, C=N; 1565, C=C; 1230, C–O–C; 1H-NMR: 0.95 (t, 3H), 1.56-164 (m, 4H), 3.20 (t, 2H), 5.43 (s, 2H), 7.35-7.70 (m, 7H), 8.33 (s, 1H); 13C-NMR: 14.3, 22.5, 33.3, 37.4, 59.8, 111.0, 116.1, 119.2, 119.9, 124.0 (2C), 125.3, 131.3, 132.1, 135.1 (2C), 138.4, 139.8, 153.0, 153.9, 159.9, 165.3; Mass: 470 (M+); Elemental Analysis, Found (Calcd.) for C22H19BrN2O3S: C, 56.01 (56.06); H, 4.0 (4.06); N, 5.95 (5.94).
3-(2-(2-(butylthio)-1H-benzo[d]imidazol-1-yl)acetyl)-6-iodo-2H-chromen-2-one (3e)
Rf: 0.69; m.p.: 15-157°C; % yield: 55; IR: 1719 & 1705, C=O; 1625, C=N; 1560, C=C; 1230, C–O–C; 1H-NMR: 0.94 (t, 3H), 1.55-165 (m, 4H), 3.20 (t, 2H), 5.44 (s, 2H), 7.32-7.71 (m, 7H), 8.34 (s, 1H); 13C-NMR: 14.4, 22.5, 33.4, 37.4, 59.9, 93.7, 111.0, 116.2, 121.4, 124.0 (2C), 124.9, 132.1, 135.2 (2C), 138.2, 138.9, 139.9, 152.9, 153.5, 159.9, 165.4; Mass: 518 (M+); Elemental Analysis, Found (Calcd.) for C22H19IN2O3S: C, 50.99 (50.98); H, 3.74 (3.69); N, 5.41 (5.40).
3-(2-(2-(butylthio)-5-methoxy-1H-benzo[d]imidazol-1-yl)acetyl)-2H-chromen-2-one (3f)
Rf: 0.66; m.p.: 165-167°C; % yield: 55; IR: 1715 & 1699, C=O; 1620, C=N; 1555, C=C; 1220, C–O–C; 1H-NMR: 0.95 (t, 3H), 1.54-163 (m, 4H), 3.19 (t, 2H), 3.88 (s, 3H), 5.41 (s, 2H), 7.30-7.70 (m, 7H), 8.33 (s, 1H); 13C-NMR: 14.3, 22.5, 33.5, 37.4, 56.8, 59.9, 101.5, 112.3, 114.1, 117.1, 119.2, 126.4, 127.4, 128.9, 129.2, 132.1, 138.3, 139.8, 153.5, 154.0, 157.2, 159.9, 165.1; Mass (m/z): 422 (M+); Elemental Analysis, Found (Calcd.) for C23H22N2O4S: C, 65.33 (65.39); H, 5.23 (5.25); N, 6.65 (6.63).
3-(2-(2-(butylthio)-5-methoxy-1H-benzo[d]imidazol-1-yl)acetyl)-6-fluoro-2H-chromen-2-one (3g)
Rf: 0.75; m.p.: 145-147°C; % yield: 55; IR: 1715 & 1695, C=O; 1622, C=N; 1560, C=C; 1224, C–O–C; 1H-NMR: 0.94 (t, 3H), 1.57-164 (m, 4H), 3.17 (t, 2H), 3.87 (s, 3H), 5.44 (s, 2H), 7.31-7.70 (m, 6H), 8.35 (s, 1H); 13C-NMR: 14.4, 22.5, 33.2, 37.4, 56.8, 59.9, 101.7, 112.4, 114.0, 115.5, 116.1, 124.7, 126.2, 127.1, 132.1, 138.3, 139.9, 149.6, 153.5, 157.1, 159.1, 159.9, 165.2; Mass: 440 (M+); Elemental Analysis, Found (Calcd.) for C23H21FN2O4S: C, 62.70 (62.72); H, 4.75 (4.81); N, 6.37 (6.36).
3-(2-(2-(butylthio)-5-methoxy-1H-benzo[d]imidazol-1-yl)acetyl)-6-chloro-2H-chromen-2-one (3h)
Rf: 0.79; m.p.: 151-153°C; % yield: 65; IR: 1695 & 1721, C=O; 1630, C=N; 1565, C=C; 1230, C–O–C; 1H-NMR: 0.95 (t, 3H), 1.56-163 (m, 4H), 3.21 (t, 2H), 3.88 (s, 3H), 5.40 (s, 2H), 7.33-7.69 (m, 6H), 8.33 (s, 1H); 13C-NMR: 14.3, 22.4, 33.4, 37.3, 56.9, 59.9, 101.8, 112.4, 114.2, 119.0, 124.5, 127.1, 127.9, 130.4, 132.1, 132.1, 138.3, 140.8, 152.1, 153.5, 157.1, 159.9, 165.1; Mass: 456 (M+); Elemental Analysis, Found (Calcd.) for C23H21ClN2O4S: C, 60.40 (60.46); H, 4.65 (4.63); N, 6.10 (6.13).
6-bromo-3-(2-(2-(butylthio)-5-methoxy-1H-benzo[d]imidazol-1-yl)acetyl)-2H-chromen-2-one (3i)
Rf: 0.68; m.p.: 161-163°C; % yield: 60; IR: 1700 & 1715, C=O; 1625, C=N; 1565, C=C; 1225, C–O–C; 1H-NMR: 0.93 (t, 3H), 1.56-165 (m, 4H), 3.18 (t, 2H), 3.87 (s, 3H), 5.41 (s, 2H), 7.35-7.70 (m, 6H), 8.34 (s, 1H); 13C-NMR: 14.3, 22.5, 33.2, 37.3, 56.8, 59.9, 101.7, 112.4, 114.2, 119.1, 119.9, 125.3, 127.4, 131.2, 132.1, 135.1, 138.3, 139.9, 153.0, 153.8, 157.1, 159.9, 165.3; Mass: 500 (M+); Elemental Analysis, Found (Calcd.) for C23H21BrN2O4S: C, 55.13 (55.10); H, 4.19 (4.22); N, 5.55 (5.59).
3-(2-(2-(butylthio)-5-methoxy-1H-benzo[d]imidazol-1-yl)acetyl)-6-iodo-2H-chromen-2-one (3j)
Rf: 0.78; m.p.: 143-145°C; % yield: 70; IR: 1700 & 1720, C=O; 1630, C=N; 1555, C=C; 1225, C–O–C; 1H-NMR: 0.94 (t, 3H), 1.55-165 (m, 4H), 3.17 (t, 2H), 3.86 (s, 3H), 5.40 (s, 2H), 7.33-7.70 (m, 6H), 8.36 (s, 1H); 13C-NMR: 14.1, 22.5, 33.3, 37.3, 56.9, 59.9, 93.9, 101.9, 112.4, 114.2, 121.3, 124.7, 127.4, 132.1, 135.1, 136.1, 138.3, 139.9, 152.5, 153.6, 157.1, 159.9, 165.4; Mass: 548 (M+); Elemental Analysis, Found (Calcd.) for C23H21IN2O4S: C, 50.35 (50.37); H, 3.85 (3.86); N, 5.13 (5.11).
Table 1 presents the antimicrobial activity and the antitubercular activity evaluation records of the compounds 1a-1b and 3a-3j.
Table 1: Antimicrobial activity records of the compounds 1a-1b and 3a-3j.
| Compounds | MIC (μg/ml) | |||||||
| S. aureus | E. faecalis | E. coli | K. pneumonia | C. albicans | P. citrinum | M. intercellulari | M. smegmatis | |
| 1a | 25 | 25 | 25 | 20 | 10 | 10 | 12.5 | 12.5 |
| 1b | 30 | 30 | 30 | 30 | 20 | 20 | 15 | 15 |
| 3a | 30 | 30 | 30 | 30 | 25 | 25 | 25 | 25 |
| 3b | 25 | 25 | 25 | 25 | 25 | 25 | 15 | 15 |
| 3c | 25 | 25 | 25 | 25 | 20 | 20 | 20 | 20 |
| 3d | 25 | 25 | 25 | 25 | 25 | 25 | 20 | 20 |
| 3e | 40 | 40 | 40 | 40 | 30 | 30 | 25 | 25 |
| 3f | 25 | 25 | 25 | 25 | 25 | 25 | 20 | 20 |
| 3g | 20 | 20 | 20 | 20 | 20 | 20 | 12.5 | 15 |
| 3h | 20 | 25 | 25 | 20 | 15 | 15 | 15 | 15 |
| 3i | 25 | 25 | 25 | 25 | 25 | 25 | 20 | 20 |
| 3j | 30 | 30 | 30 | 30 | 20 | 20 | 25 | 25 |
| Ofloxacin | 20 | 20 | 20 | 15 | – | – | – | – |
| Ketoconazole | – | – | – | – | 10 | 10 | – | – |
| Isoniazid | – | – | – | – | – | – | 10 | 10 |
| Control | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
All values had p <0.05.
Table 2 presents the antioxidant activity records of the compounds 1a-1b and 3a-3j.
Table 2: Antioxidant activity record of 1a-1b and 3a-3j
| Compound | % Antioxidant Activity (N = 3) at different concentration (μg/ml) | Calculation of IC50 (μg/ml) By Linear Regression (Y = mx + c) | ||||
| 5 | 10 | 15 | 20 | 25 | ||
| 1a | 18.75±0.15 | 35.09±0.18 | 53.85±0.58 | 71.10±0.10 | 91.16±0.14 | 13.90 (86.18%) |
| 1b | 23.07±0.17 | 40.14±0.22 | 56.99±0.30 | 78.80±0.50 | 93.99±0.72 | 12.62 (94.92%) |
| 3a | 17.42±0.44 | 27.70±0.42 | 42.82±0.42 | 62.10±0.19 | 75.85±0.30 | 16.59 (72.21%) |
| 3b | 23.10±0.10 | 36.15±0.18 | 52.10±0.18 | 64.15±0.90 | 76.15±0.30 | 14.88 (80.51%) |
| 3c | 22.47±0.15 | 37.70±0.50 | 55.80±0.50 | 66.09±0.60 | 79.30±0.70 | 14.20 (84.36%) |
| 3d | 15.67±0.40 | 24.15±0.50 | 39.90±0.18 | 54.15±1.10 | 67.70±0.40 | 18.61 (64.37%) |
| 3e | 17.25±0.18 | 28.15±0.40 | 43.65±0.60 | 61.10±0.50 | 77.92±0.30 | 16.42 (72.95%) |
| 3f | 24.41±0.12 | 39.15±0.14 | 57.19±0.20 | 67.15±0.10 | 81.90±0.30 | 13.61 (88.02%) |
| 3g | 18.15±0.14 | 28.40±0.30 | 43.80±0.36 | 60.02±0.60 | 71.10±0.50 | 17.07 (70.18%) |
| 3h | 16.13±0.16 | 27.10±0.40 | 42.09±0.50 | 54.10±0.40 | 69.70±0.10 | 18.05 (66.37%) |
| 3i | 21.66±0.20 | 35.30±0.40 | 49.15±0.49 | 63.30±0.39 | 77.10±0.40 | 15.25 (78.55%) |
| 3j | 23.15±0.10 | 38.40±0.30 | 56.10±0.30 | 73.42±0.30 | 91.30±0.80 | 13.11 (91.38%) |
| Ascorbic Acid | 24.22±0.51 | 43.41±0.23 | 58.90±0.30 | 82.51±0.15 | 95.98±0.20 | 11.98 (100%) |
| Control | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
All values had p <0.05.
Discussion
The compounds of the present work were synthesized as per Figure 1. The compound of the formula 1a-1b and 2a-2e were prepared according to the prior art processes.1,16,17 The reaction of appropriate compound 1a-1b with another appropriate compound 2a-2e in acetone in the existence of sodium carbonate provided the compounds 3a-3j. These compounds were recrystallized from ethanol and were characterized by their physical and spectral records. These data supported the assigned structures and are presented in details in the result part.
The data of the antimicrobial activity showed that the compound 3g and 3h had equal MIC values against S. aureus concerning ofloxacin; the compound 3g had equal MIC value against E. faecalis and E. coli concerning ofloxacin; the compound 1a had equal MIC value against C. albicans and P. citrinum concerning ketoconazole; not any compound demonstrated equipotent MIC value against K. pneumonia concerning ofloxacin; the compounds 1a and 3g displayed closest MIC value against M. intercellulari and M. smegmatis. The SAR study demonstrated that the substitution of the iodine provides least potent compounds, wherein the compounds bearing –F, -Cl, and –Br groups at C-6 of the coumarin ring along with the –OCH3 group at C-5 of the benzimidazole ring provide potent compounds. It is assumed that the –F, -Cl, -Br, and the –OCH3 groups make the compounds more lipophilic, which may be responsible for the better antimicrobial activity the compounds possessing these groups.21 There is also a possibility that increasing the carbons in the 2-thiobutyl chain may increase the potency of these type of compounds. Using the same concept in compounds 1a and 3g, better anti-tubercular agents may also be prepared because the cell wall of the Mycobacterium is highly lipophilic.18
The antioxidant activity data provided that the compound 1b had the highest antioxidant activity of 94.92%, and the compound 3d had the least antioxidant activity of 64.37% with respect to the standard ascorbic acid. Other compounds showed moderate antioxidant activity concerning ascorbic acid. It is expected that the tested compounds had moderate antioxidant activity because of the structural similarity of the coumarin ring with flavonoid structure.22 There is a possibility that replacement of the coumarin ring with another close flavonoid structure may provide compounds having better antioxidant activity.
Conclusion
It has been concluded that replacing the 2-butylthio group with 2-pentylthio or 2-hexylthio substituents may provide more potent antimicrobial compounds than the compounds of the present report. The replacement of the coumarin structure with another close flavonoid structure may provide better antioxidant compounds. Therefore, replacement of the 2-butylthio group and the coumarin moiety with the suggested group is recommended. Accordingly, further modification of these compounds is in progress in our laboratory.
Acknowledgment
The author would like to thank Northern Border University for providing facilities to carry out this research work.
Conflicts of Interest
There are no conflicts of interest.
References
- Ansari M.I., Khan, S.A. Chem. Res. 2017; 26(7), 1481-1496.
- Imran M., Bakht M.A., Samad A., Abida. J. Pharm. Res. 2017; 16(5), 1147-1155.
- Yeung A.W.K., Tzvetkov N.T., El-Tawil O.S., Bungǎu S.G., Abdel-Daim M.M., Atanasov A.G. Med. Cell. Longev. 2019; 8278454.
- Fitzmaurice S.D., Sivamani R.K., Isseroff R.R. Skin Pharmacol. Physiol. 2011; 24(3), 113-126.
- Singh L.R., Avula S.R., Raj S., Srivastava A., Palnati G.R., Tripathi C.K.M., Pasupuleti M., Sashidhara K.V. Antibiot. (Tokyo). 2017; 70(9), 954-961.
- Liu L., Shen Y.F., Hu Y., Lu J.F. Fish Shellfish Immunol. 2018; 83, 386-396.
- Shingalapur R.V., Hosamani K.M., Keri R.S., Hugar M.H. J. Med. Chem. 2010; 45(5), 1753-1759.
- Lee S.O., Choi S.Z., Lee J.H., Chung S.H., Park S.H., Kang H.C., Yang E.Y., Cho H.J., Lee K.R. Pharm. Res. 2004; 27(12), 1207-1210.
- Zhang Y., Xu J., Li Y., Yao H., Wu X. Biol. Drug Des. 2015; 85(5), 541-548.
- Razavi B.M., Arasteh E., Imenshahidi M., Iranshahi M. Iran J. Basic Med. Sci. 2015; 18(2), 153-158.
- Liu H., Wang Y., Sharma A., Mao R., Jiang N., Dun B., She J.X. Anticancer Drugs. 2015; 26(6), 667-77.
- Sharma R., Bali A., Chaudhari B.B. Med. Chem. Lett. 2017; 27(13), 3007-3013.
- Nagamallu R., Srinivasan B., Ningappa M.B., Kariyappa A.K. Med. Chem. Lett. 2016; 26(2), 690-694.
- Imran M., Abida, Khan S.A. J. Pharm. Res. 2015; 14(7), 1265-1272.
- Imran M., Abida, Alsalman A.J. J. Pharm. Res. 2016; 15(2), 393-404.
- Imran M., Nayeem N., El-Feky S.A. Am. J. Pharm. Sci. 2017; 4(04), 840-851.
- Alanazi A.H.G., Alam M.T., Imran M. Am. J. Pharm. Sci. 2017; 4(04), 926-936.
- Imran M., Bawadekji A., Ali M.A. J. Pharm. Bio. Chem. Sci. 2018; 9(5), 2413-2419.
- Antolovich M., Prenzler P.D., Patsalides E., McDonald S., Robards K. Analyst. 2002; 127(1), 183-198.
- Salar U., Khan K.M., Chigurupati S., Syed S., Vijayabalan S., Wadood A., Riaz M, Ghufran M., Perveen S. Chem. 2019; 15(1), 87-101.
- Göker H., Alp M., Yildiz S. Molecules. 2005; 10(11), 1377–1386.
- Lotito S.B., Frei B. Med. 2006; 41(12), 1727-1746.

This work is licensed under a Creative Commons Attribution 4.0 International License.









