Analgesic and Anxiolytic Activities of Achillea Biebersteinii: Evidence for the Involvement of GABAergic Systems
Manal Ahmad Abbas*1, Sahar Majdi Jaffal2 and Belal Omar Al-Najjar3
1Faculty of Pharmacy, Al-Ahliyya Amman University, 19328, Amman, Jordan.
2Department of Biological Sciences, Faculty of Science, The University of Jordan, 11942 Amman.
Corresponding Author E-mail: m.abbas@ammanu.edu.jo
DOI : http://dx.doi.org/10.13005/ojc/350426
Article Received on : 06-06-2019
Article Accepted on : 14-07-2019
Article Published : 24 Jul 2019
Achillea biebersteinii (Asteraceae) is used in traditional medicine for treating abdominal pain, menstrual pain and headache. The analgesic, antidepressant and anxiolytic activities of this plant were studied. Moreover, molecular docking technique was used for plant constituents to determine their energy of binding against GABAA and GABAB receptors. A. biebersteinii decreased flinching in early and late phases of formalin test and increased the time in hot plate test. In forced swimming test, no difference in immobility time was found. In open field test, high doses decreased the crossed lines number and rearing behavior. A. biebersteinii increased the time that the animals spent in the open arm side of elevated plus maze apparatus. Both bicuculline and SCH 50911 reversed A. biebersteinii action. Lavndulyl-2-methylbutanoate and sesquisabinene hydrate, showed the lowest binding energies for both GABAA and GABAB receptors. In conclusion, A. biebersteinii exerted analgesic, anxiolytic but no antidepressant activity. Its effect involved interaction with GABAA and GABAB systems.
KEYWORDS:Achillea Biebersteinii; Analgesic; Anxiolytic; Antidepressant; GABA Receptor; Molecular Docking
Download this article as:| Copy the following to cite this article: Abbas M. A, Jaffal S. M, Al-Najjar B. O. Analgesic and Anxiolytic Activities of Achillea Biebersteinii: Evidence for the Involvement of GABAergic Systems. Orient J Chem 2019;35(4). |
| Copy the following to cite this URL: Abbas M. A, Jaffal S. M, Al-Najjar B. O. Analgesic and Anxiolytic Activities of Achillea Biebersteinii: Evidence for the Involvement of GABAergic Systems. Orient J Chem 2019;35(4). Available from: https://bit.ly/2JOtqzn |
Introduction
Disorders of depression and anxiety are considered the most common psychiatric and behavioral problems.1 World Health Organization (WHO) reports show that the number of people suffering from anxiety disorders worldwide reaches 400 million.2 Recently, discovery of new anxiolytic and antidepressant drugs have gained great attention due to the impact of depression on the life of patients as it can lead to suicidal attempts.3 Furthermore, side effects of the available anxiolytic and antidepressant drugs are serious. For example, the anxiolytic drugs benzodiazepines cause confusion, tolerance amnesia, aggression, physical dependence and decline of cognitive functioning as well as psychomotor impairment.4 Antidepressants can lead to insomnia as well as gastrointestinal and sexual problems.1 This can harm the patient or lead to the discontinuation of medication.
The use of medicines is preferred in many aspects due to the advantages of being inexpensive, more culturally acceptable and because they have less side effects to the human body5,6; It is well-established that botanical natural products in traditional medicine are efficient in treating anxiety and other similar disorders in humans.7 For example, many essential oils of plants have anxiolytic and analgesic effects.8 In addition, the well-known analgesic drugs morphine, codeine and capsaicin are all of plant origin.9 Therefore, the search for safe medicines from natural products is a remarkable challenge for current research.10
The genus Achillea comprises 85 species. Many of Achillea species are considered endemic types to the Middle East and Europe.11 These species are used in cosmetics, fragrances and as folk remedies for different purposes such as wounds and abdominal pain. Achillea biebersteinii Afan. (Asteraceaeis) is used to treat abdominal pain, menstrual pain, stomachache, headache, wounds, cold and haemorrhoids12,13; A recent study has reported that A. biebersteinii posseses anxiolytic activity in scopolamine-induced amnesic rat model.14 It is well known that anxiolytic drugs may interact with and influence brain γ-aminobutyric acid (GABA) levels and neurotransmission. Interaction with GABA receptors was reported for several Achillea spp. such as for A fragrantissima.15 However, it is not known yet if such an interaction exists between A. biebersteinii and GABAergic systems. This study investigates this aspect and tests whether this interaction mediates the previously reported analgesic activity of A. biebersteinii.
Materials and Method
Plant extraction and identification of chemical constituents
A. biebersteinii flowers were collected in the wild from Salt region (Jordan) during May 2015. The classification of the plant was confirmed by Prof. Barakat Abu-Irmaileh, Faculty of Agriculture/The University of Jordan. A voucher specimen was preserved at the Laboratory of Graduate Studies in Al-Ahliyya Amman University (Ast#5-2015).
Flower extract was prepared by grinding the flowers and soaking them in 96% methanol at room temperature for 72 hr. The solvent was evaporated at low pressure at a temperature not exceeding 45°C. The dry extract was stored at -20°C. On the day of experiment, the extract was dissolved in sterile saline before use. Gas chromatography-mass spectrometry (GC-MS) analysis of the flower extract was performed. The details of the analysis method are described in our previous work.16 Also, the chemical constituents were fully identified. Briefly the identified compounds were: thymol (0.8%), p-cymene (1.11 %), linalool (0.35%), trans-sabinene ( 0.9%), terpinene-4-ol (0.51 %), ascaridole (43.22 %), iso-ascaridole (37.87 %), alpha-terpenine (0.2%), lavndulyl-2- trans-sesquisabinene hydrate (1.2 %), pentacosane(N-) (1.78 %), nerol (0.48 %), hexadecene (0.7 %), 1.8-cineol (0.8%), sabinene (0.2%), eugenol (1.13 %), hexadecenoic acid (0.67 %), methylbutanoate (1.01%), cis-piperitone epoxide (0.44 %), gamma-eudesmol (0.8 %) trans-piperitone epoxide ( 0.9%) tricosane (N-) (1.8 %), carvacrol (1.6 %), hexadecenoic acid methyl ester ( 0.6%), and octadecene (0.38 %).
Drugs
Butoxamine and bicuculline were purchased from Sigma-Aldrich, US. Clomipramin hydrochloride was obtained from Novartis, Switzerland. Diclofenac sodium from Diclopan, Taiwan. Indomethacin was purchased from DAD company, Jordan and diazepam was brought from Medochemie LTD, Cyprus. Yohimbine and SCH 50911 (2S)-(+)-5,5-dimethyl-2-morpholineacetic acid) were from Tocris Bioscience, UK. All drugs were dissolved in sterile normal saline except bicuculline that was suspended in 1% carboxy methyl cellulose.
Experimental Animals
BALB/c mice were brought from the animal room at Al-Ahliyya Amman University, Amman, Jordan. Female mice weighing 20-23g were used. Animals were kept at 23±2oC with a cycle of light and dark (12 hr each). Water and food pellets were provided according to the standards of the animal care. Adaptation of mice to the laboratory conditions was allowed for at least 2 hrs before tests. All aspects of the research described here were carried out in accordance with the Jordanian Animal Welfare By-Law No. (11) published in 2010. The research was accepted by the ethical committee at Al-Ahliyya Amman University and was given the ethical approval number AAU-2/4/2018.
Paw-licking Test
Mice (9 animals per group) were injected intraperitoneally (i.p) with vehicle (sterile normal saline), 300 mg/kg, 400 mg/kg, 500 mg/kg A. biebersteinii methanolic flower extract or 50 mg/kg indomethacin (positive control) 30 min before formalin injection. The doses of antagonists and injection time were selected based on previous studies as the following: Yohimbine 1 mg/kg,17 butoxamine 8 mg/kg,18 bicuculline 4 mg/kg.19 Yohimbine, butoxamine and bicuculline were administered 15 min before extract while SCH 50911 (10 mg/kg) was given 30 min before formalin injection according to Rawls et al. 20 Paw licking test was carried out as in the method of Hunskaar et al.21 20 µl formalin (2.5%) was administered to the left hind paw of the animal via intraplantar injection. The behavior of animals was recorded by video. The time spent in flinching and licking responses was measured in early phase (the first 5 min) and late phase (the last 5 min) of formalin test (30 minutes).
Hot Plate Test
Mice were divided into different groups with 12 mice in each group. Plant extract or vehicle were given 30 min before the test. Bicuculline (4 mg/kg) was administered 15 min before extract while SCH 50911 (10 mg/kg) was given 30 min before extract. Each animal was placed into a glass beaker on a hot plate that was kept at 55±1°C. The time between starting the test and its first jump was considered as a reflection of the latency of pain. To avoid tissue damage in mice, a cutoff time of 60s was used.
Forced Swimming Test (FST)
The test was performed according to the method published in Porsolt et al. 22 with some modifications. Plant extract or vehicle were given half an hour before the test. Mice (10 per group) were forced to, individually, in 10 cm wide glass container. The 25 cm height container was filled with water (25±1°C). The swimming during a 5 min test was recorded in a noise and light-controlled room. The immobility time was recorded for 3 min after 2 min from the beginning of swimming. Each animal was considered to be immobile when it stopped struggling or only made the necessary movements for floating with its head above water.
Open-Field Test (OFT)
The ambulatory behavior was tested in the OFT according to Royce.23 Mice were adapted to the laboratory conditions for about 2 hrs. Plant extract or vehicle were given 30 min before the test. Bicuculline (4 mg/kg) was administered 15 min before extract while SCH 50911 (10 mg/kg) was given 30 min before extract. The open–field apparatus consisted of a 30 cm x 40 cm and 20 cm high. The floor was partitioned to 20 equal squares to count. The number of squares that each animal crossed and the times of rearing (i.e. when the mouse stood on the hind paws) was recorded during a period of 3 min. The floor of the apparatus was cleaned between trials using 70% ethanol to eliminate the effect of odors left by previous mice. The test was carried out at room temperature (23 ± 2°C) in a light and noise-controlled room. Ten mice were used per group.
Elevated Plus Maze Test
Each animal was placed facing one of the closed arms of the elevated plus maze (50 cm above ground) and tested only once. Each closed and open arm was 10 cm long and 5 cm wide. Plant extract or vehicle were given 30 min before the test. Bicuculline (4 mg/kg) was administered 15 min before extract while SCH 50911 (10 mg/kg) was given 30 min before extract. Mice were video recorded by camera for 5 min. Eight animals were used per group. Entry into an arm was considered when all four feet of the mouse enter into that arm. The time spent, totally, in both arms (open and closed arms) was recorded. The percentage of time spent in open arm was calculated (time in which the animal stays in open arm/ total time of the test * 100).
Molecular Docking
The following software packages were utilized in this project:
ACD/ChemSketch, (acdlabs.com)24
Autodock 4.225
Biovia Discovery Studio visualizer (http://accelrys.com)
To understand the mechanism of action for plant constituents, docking simulations were performed on CryoEM structure of of GABAA (PDB code: 6HUO26) and X-Ray crystal structure of GABAB (PDB code: 4MS427). Plant constituents have been drawn and saved as mol2 files by ChemSketch software and then converted to pdb files. Ligand files in pdb format were prepared by AutoDockTools. Each compound was opened seperately, charges were added, and all hydrogen atoms were merged. Molecular docking simulations of the compounds were performed utilizing AutoDock 4.2. Kollman and Gasteiger charges were added to both proteins and plant compounds respectively. A set of grid maps were created, using AutoGrid 4 (The Scripps Research Institute, San Diego, CA, USA). A grid box was then utilized to select which area of the protein structure to be mapped. The size of the box was set to 15, 15 and 15 Å (x, y and z, respectively). Lamarckian genetic algorithm (LGA) was used for optimizing and minimizing energy during docking simulation.
Results and Discussion
Paw-Licking Test
In paw licking test, all tested doses of A. biebersteinii extract reduced flinching reactions and paw licking in early and late phases of formalin test (Figures 1a & b). The percentage inhibition of flinching behaviour of indomethacin-treated mice (50 mg/kg) was not statistically different from that of A. biebersteinii extracts-treated group in the two phases of formalin test (early and late). Yohimbine and butoxamine did not abolish the effect of A. biebersteinii extract while both bicuculline and SCH 50911 reversed its action only in the late phase of formalin test (Figure 1b).
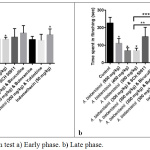 |
Figure 1: Formalin test a) Early phase. b) Late phase. |
*Significantly different from the control.
**Significant difference between A. biebersteinii 500 mg/kg and SCH 50911 & A. biebersteinii 300 mg/kg. ***Significant difference between A. biebersteinii 500 mg/kg and bicuculline & A. biebersteinii 300 mg/kg.
In our previous work, the antinociceptive activity of A. biebersteinii was studied in 3 pain models: tail flick, writhing test and formalin test. In the current investigation, the possible involvement of GABA receptors as a mechanism by which A. biebersteinii extract exerted its action was studied in chemical (formalin test) and thermal model (hot plate test). The results of this study indicate that the action of A. biebersteinii is mediated by interaction with both GABAA and GABAB systems as it was blocked by bicuculline and SCH 50911, respectively. On the other hand, A. biebersteinii flower extract analgesic action was not mediated by interaction with α2 adrenergic receptors as the action of this plant was not blocked by yohimbine nor with β2 adrenergic receptors as butoxamine didn’t abolish the effect of this plant.
The methanolic flower extract of A. biebersteinii reduced flinching behavior in both early and late phases of formalin test. Similar results were obtained by A. wilhelmsii C. Koch methanolic extract28 and the aqueous extract of A. millefolium flowers29 that reduced significantly acute and chronic pain in formalin test. In comparison, A. nobilis L. subsp. neilreichii extract caused inhibition of licking the hind-paw during the late phase only.30 On the other hand, the effect of aerial parts alcoholic extract (without flowers) of A. millefolium extract had no effect in early or late phases of formalin test.31
Hot Plate Test
In hot plate test, the highest dose (500 mg/kg) of A. biebersteinii extract increased latency at 30 min. Both bicuculline and SCH 50911 reversed the effect of A. biebersteinii in this test (Figure 2).
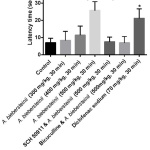 |
Figure 2: Results of hot-plate test. |
*Significantly different from the control.
**Significant difference between A. biebersteinii 500 mg/kg and SCH 50911 & A. biebersteinii 500 mg/kg. ***Significant difference between A. biebersteinii 500 mg/kg and bicuculline & A. biebersteinii 500 mg/kg.
In the current work, 500 mg/kg dose of A. biebersteinii was effective after 30 min in hot plate test. Similar results were obtained by A. nobilis extract (400 mg/kg) that increased the latency to hot-plate test when measured at 60 and 90 min after injection.30 Also, the non-polar extract A. fragrantissima produced dose-dependent analgesic effect in mice in hot plate assay.32 On the other hand, the hydroalcohol extracts of 500 and 1000 mg/kg A. millefolium had not any effect in the hot plate.31 Similar to formalin test, the mechanism of analgesic effect of A. biebersteinii in hot plate test involved an interaction with GABAA and GABAB systems as it was blocked by bicuculline and SCH 50911, respectively. Up to our best knowledge, this is the first report showing an interaction of A. biebersteinii with GABA receptors as a mechanism for its analgesic action.
Forced Swimming Test
In forced swimming, no statistically significant difference was found between A. biebersteinii extract-treated mice and control group. Immobility time in seconds was 159.3±9.0, 163.7±16.4, 161.8±8.7, 165.4±11.2 for control, 300 mg/kg, 400 mg/kg and 500 mg/kg, A. biebersteinii extract respectively. Clomipramin hydrochloride (standard drug) increased significantly immobility time (119.1±14.5 sec).
In this research, the antidepressant activity of the methanolic extract of A. biebersteinii was studied. No antidepressant action was found. A recent study reported an antidepressant activity of the volatile oil extracted from A. biebersteinii flowers in FST of scopolamine administered rats.14 This contradicts with the results of the present study in which no increase in immobility time was observed. This contradiction can be explained by the presence of different constituents in the volatile oil and the methanolic extract (containing polar compounds mainly) and the difference in the animal model used in the two studies. It should be noted that this plant is taken as herbal tea containing mostly polar compounds; rather than oil, in folk medicine.
Open Field Test
In open field test, the high doses (400 and 500 mg/kg) decreased the number of lines crossed as well as rearing behavior (Figures 3a, b). SCH 50911 and bicuculline reversed the action of A. biebersteinii in this test (Figure 3a, b).
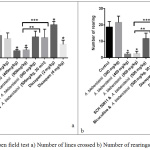 |
Figure 3: Open field test a) Number of lines crossed b) Number of rearings. |
*Significantly different from the control.
**Significant difference between A. biebersteinii 500 mg/kg and SCH 50911 & A. biebersteinii 500 mg/kg. ***Significant difference between A. biebersteinii 500 mg/kg and bicuculline & A. biebersteinii 500 mg/kg.
OFT is a common method to measure anxiety-like and sedation behaviors.33,34 High number of line crosses indicates increased locomotion while the increase in rearing behavior indicates exploration and both refers to a lower degree of anxiety 34. In this study the 400 and 500 mg/kg doses of the methanolic extract of A. biebersteinii decreased number of lines crossed, indicative of a possible sedative effect at these high doses. The lower dose (300 mg/kg), which was effective in formalin test, exerted no sedative effects in open field test.
In a previous work, the main components detected in A. biebersteinii flower extract were ascaridole and iso-ascaridole with 43.22% and 37.87%, respectively. In addition to p-cymene, carvacrol, lavndulyl-2-methylbutanoate, trans-sesquisabinene hydrate, tricosane (N-), pentacosane(N-) and eugenol.16 Okuyama et al.35 found that ascaridole has analgesic effect, prolongation of anesthesia and sedative effect as it reduced locomotor activity produced by methamphetamine. This can explain the findings that the high doses of A. biebersteinii (400 and 500 mg/kg) produced sedative effect as indicated by re the reduced locomotor activity in open field test. Similarly, essential oils of A. umbelata decreased rearings and motor activity in the open-field test.36
In open field test, A. biebersteinii extract decreased rearing behavior and number of lines crossed. The action was mediated by interaction with GABAA and GABAB systems as it was blocked by bicuculline and SCH 50911, respectively. Rearing activity is used an index of the exploratory behavior and is associated with the electrical activity of hippocampus,37 with synergistic interaction between cholinergic and GABAergic systems in this activity.38
Elevated Plus Maze Test
In elevated plus maze test A. biebersteinii flower extract (400 mg/kg) increased the time spent in open arm. The percentage of time spent in open arm was 0.2%, 1%, 5.2%, 1.5% and 22.4% for the control, 300 mg/kg, 400 mg/kg, 500 mg/kg of A. biebersteinii extract and 4 mg/kg diazepam, respectively. Both bicuculline and SCH 50911 reversed the action of A. biebersteinii in this test (Figure 4).
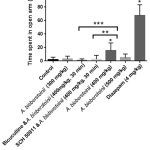 |
Figure 4: Elevated plus maze test. |
*Significantly different from the control.
**Significant difference between A. biebersteinii 400 mg/kg and SCH 50911 & A. biebersteinii 400 mg/kg.
***Significant difference between A. biebersteinii 400 mg/kg and bicuculline & A. biebersteinii 400 mg/kg.
In the present study, A. biebersteinii flower extract (400 mg/kg) increased the time spent in open arm indicating that this plant has anxiolytic effect. Up to our best knowledge this is the first report of such findings. The interaction of A. biebersteinii with GABAergic systems suggests that this plant may have anticonvulsant activity. Future studies may investigate this aspect.
Molecular Docking
Plant constituents (Figure 5) were successfully docked against GABAA and GABAB receptors, and the results are shown in Table 1. Based on the energy results, most of the compounds showed good binding energies, ranged between -5.26 to -7.15 and -5.55 to -8.59 Kcal/mol for GABAA and GABAB, respectively. Both compounds, Lavndulyl-2-methylbutanoate and Sesquisabinene hydrate, showed the lowest binding energies for both receptors. The intermolecular interactions of the best docked compounds are displayed in Figures 6 and 7. The intermolecular interactions against GABAA are illustrated in Figure 6, in which Lavndulyl-2-methylbutanoate performs two hydrogen bond interactions with Ser205 and Thr207, whereas Sesquisabinene hydrate performs only one hydrogen bond interaction with Ser159. Lavndulyl-2-methylbutanoate additionally performs hydrophobic interactions with Tyr58, Phe77, Ala79 and Tyr210. Whereas, Sesquisabinene hydrate interact with Phe77, Ala79, Phe100, Tyr160 and Tyr210. On the other hand, Figure 7 shows the intermolecular interactions for Lavndulyl-2-methylbutanoate and Sesquisabinene hydrate against GABAB receptor. Lavndulyl-2-methylbutanoate found to form one hydrogen bond interaction with Glu251 and hydrophobic interactions with His170, Trp65, Val201, Phe202 and Tyr250. However, Sesquisabinene hydrate shows to interact with Ser130 and Gly151 by hydrogen bond interactions. Moreover, it performs hydrophobic interactions with Trp65, Pro66, His170, Trp278 and Tyr279.
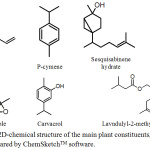 |
Figure 5: 2D-chemical structure of the main plant constituents, prepared by ChemSketchTM software. |
Table 1: The lowest binding energies obtained from AutoDock 4.2 for plant constituents against GABAA and GABAB receptors.
| Compound | Lowest Free Energy of Binding (Kcal/mol) | |
| GABAA | GABAB | |
| Ascaridole | -6.76 | -6.49 |
| Carvacrol | -5.97 | -5.92 |
| P-cymene | -5.47 | -5.55 |
| Eugenol | -5.26 | -5.87 |
| Iso-ascaridole | -5.83 | -6.08 |
| Lavndulyl-2-methylbutanoate | -6.73 | -8.10 |
| Sesquisabinene hydrate | -7.15 | -8.59 |
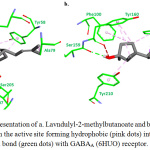 |
Figure 6: Stick representation of a. Lavndulyl-2-methylbutanoate and b. Sesquisabinene hydrate in the active site forming hydrophobic (pink dots) interactions and hydrogen bond (green dots) with GABAA (6HUO) receptor. |
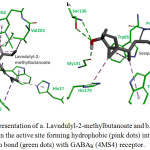 |
Figure 7: Stick representation of a. Lavndulyl-2-methylbutanoate and b. Sesquisabinene hydrate in the active site forming hydrophobic (pink dots) interactions and hydrogen bond (green dots) with GABAB (4MS4) receptor. |
In conclusion, A. biebersteinii exerted analgesic, anxiolytic but no antidepressant action. Its effect in late phase of formalin test, hot plate, elevated plus maze and open field tests involved interaction with GABAA and GABAB systems. Lavndulyl-2-methylbutanoate and sesquisabinene hydrate are the active constituents most probably responsible for this activity. Future studies may test the biological activities of these compounds in vivo.
Acknowledgements
This work was published with the support of Al-Ahliyya Amman University.
Conflict of Interest
There is no conflict of interest.
References
- He, D.; Wang, X.; Zhang, P.; Luo, X.; Li, X.; Wang, L.; Li, S.; Xu, Y., Evaluation of the anxiolytic and antidepressant activities of the aqueous extract from Camellia euphlebia Merr. ex Sealy in mice. Evidence-Based Complementary and Alternative Medicine 2015, 2015.
- Sadock, B. J.; Sadock, V. A., Kaplan and Sadock’s synopsis of psychiatry: Behavioral sciences/clinical psychiatry. Lippincott Williams & Wilkins: 2011.
- Ferrari, A. J.; Charlson, F. J.; Norman, R. E.; Patten, S. B.; Freedman, G.; Murray, C. J.; Vos, T.; Whiteford, H. A., Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS medicine 2013, 10 (11), e1001547.
- Rout, S. K.; Kar, D., Sedative, anxiolytic and anticonvulsant effects of different extracts from the leaves of Ipomoea carnea in experimental animals. Int J Drug Dev Res 2013, 5 (2), 232-243.
- Pal, S. K.; Shukla, Y., Herbal medicine: current status and the future. Asian Pacific journal of cancer prevention : APJCP 2003, 4 (4), 281-8.
- Rivera, J.; Loya, A.; Ceballos, R., Use of herbal medicines and implications for conventional drug therapy medical sciences. Altern Integ Med 2013, 2 (6), 1-6.
- Barnes, P. M.; Bloom, B.; Nahin, R. L., Complementary and alternative medicine use among adults and children; United States, 2007. 2008.
- de Oliveira Júnior, W. M.; Benedito, R. B.; Pereira, W. B.; de Arruda Torres, P.; Ramos, C. A. F.; Costa, J. P.; da Rocha Tomé, A.; de Sousa, D. P.; de Freitas, R. M.; de Fatima Formiga Melo Diniz, M., Farnesol: antinociceptive effect and histopathological analysis of the striatum and hippocampus of mice. Fundamental & clinical pharmacology 2013, 27 (4), 419-426.
- Vij, V. V.; Sharma, V.; Kumar, P.; Deshmukh, R., Herbs as positive modulator in neuropathic pain and their antinociceptive effect.
- Fedotova, J.; Kubatka, P.; Büsselberg, D.; Shleikin, A. G.; Caprnda, M.; Dragasek, J.; Rodrigo, L.; Pohanka, M.; Gasparova, I.; Nosal, V., Therapeutical strategies for anxiety and anxiety-like disorders using plant-derived natural compounds and plant extracts. Biomedicine & Pharmacotherapy 2017, 95, 437-446.
- Ali, S. I.; Gopalakrishnan, B.; Venkatesalu, V., Pharmacognosy, phytochemistry and pharmacological properties of Achillea millefolium L.: A review. Phytotherapy research 2017, 31 (8), 1140-1161.
- Akkol, E. K.; Koca, U.; Pesin, I.; Yilmazer, D., Evaluation of the wound healing potential of Achillea biebersteinii Afan.(Asteraceae) by in vivo excision and incision models. Evidence-Based Complementary and Alternative Medicine 2011, 2011.
- Tetik, F.; Civelek, S.; Cakilcioglu, U., Traditional uses of some medicinal plants in Malatya (Turkey). J Ethnopharmacol 2013, 146 (1), 331-46.
- Akbaba, E.; Hassan, S.; Mohammed Sur, T.; Bagci, E., Memory Enhancing, Anxiolytic and Antidepressant Effects of Achillea biebersteinii (Asteraceae) Essential Oil on Scopolamine-Induced Rats. Journal of Essential Oil Bearing Plants 2018, 21 (3), 825-839.
- Mudawi, M. M.; El-wahab, M. F. A.; Yassin, A. Y.; Habeballa, R. S.; Alshehri, M. M., Evaluation of Anticonvulsant Activity and HPLC–DAD Profiling of Achillea fragrantissima (Gaisoom) Extracts Growing in Saudi Arabia. Asian Journal of Pharmaceutical Research and Health Care 2017, 9 (3), 92-100.
- Jaffal, S. M.; Abbas, M. A., Antinociceptive action of Achillea biebersteinii methanolic flower extract is mediated by interaction with cholinergic receptor in mouse pain models. Inflammopharmacology 2018.
- Rodrigues, A. L.; Rosa, J. M.; Gadotti, V. M.; Goulart, E. C.; Santos, M. M.; Silva, A. V.; Sehnem, B.; Rosa, L. S.; Goncalves, R. M.; Correa, R.; Santos, A. R., Antidepressant-like and antinociceptive-like actions of 4-(4′-chlorophenyl)-6-(4”-methylphenyl)-2-hydrazinepyrimidine Mannich base in mice. Pharmacology, biochemistry, and behavior 2005, 82 (1), 156-62.
- Lopez-Alvarez, V. M.; Puigdomenech, M.; Navarro, X.; Cobianchi, S., Monoaminergic descending pathways contribute to modulation of neuropathic pain by increasing-intensity treadmill exercise after peripheral nerve injury. Experimental neurology 2018, 299 (Pt A), 42-55.
- Wang, S.; Wang, C.; Peng, D.; Liu, X.; Wu, C.; Guo, P.; Wei, J., Agarwood Essential Oil Displays Sedative-Hypnotic Effects through the GABAergic System. Molecules (Basel, Switzerland) 2017, 22 (12).
- Rawls, S. M.; Tallarida, R. J.; Kon, D. A.; Geller, E. B.; Adler, M. W., GABAA receptors modulate cannabinoid-evoked hypothermia. Pharmacology, biochemistry, and behavior 2004, 78 (1), 83-91.
- Hunskaar, S.; Fasmer, O. B.; Hole, K., Formalin test in mice, a useful technique for evaluating mild analgesics. Journal of neuroscience methods 1985, 14 (1), 69-76.
- Porsolt, R.; Bertin, A.; Jalfre, M., Behavioral despair in mice: a primary screening test for antidepressants. Archives internationales de pharmacodynamie et de therapie 1977, 229 (2), 327-336.
- Royce, J. R., On the construct validity of open-field measures. Psychological bulletin 1977, 84 (6), 1098.
- ACD/Labs, ACD/Structure Elucidator. Advanced Chemistry Development, Inc.: Toronto, ON, Canada, 2019; Vol. 2018.1.
- Morris, G. M.; Huey, R.; Lindstrom, W.; Sanner, M. F.; Belew, R. K.; Goodsell, D. S.; Olson, A. J., AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. Journal of computational chemistry 2009, 30 (16), 2785-2791.
- Masiulis, S.; Desai, R.; Uchanski, T.; Serna Martin, I.; Laverty, D.; Karia, D.; Malinauskas, T.; Zivanov, J.; Pardon, E.; Kotecha, A.; Steyaert, J.; Miller, K. W.; Aricescu, A. R., GABAA receptor signalling mechanisms revealed by structural pharmacology. Nature 2019.
- Geng, Y.; Bush, M.; Mosyak, L.; Wang, F.; Fan, Q. R., Structural mechanism of ligand activation in human GABA(B) receptor. Nature 2013, 504 (7479), 254-9.
- Sarshogh, M.; Monajemi, R.; Amjad, L., Analgesic and Anti-Inflammatory effects of methanolic extracts of immature flowers Achillea wilhelmsii C. Koch in mice (Balb/c). Scinzer. J Agric Biol Sci. 2016, 2, 28-32.
- Noureddini, M.; Rasta, V., Analgesic Effect of aqueous extract of Achillea millefolium L. on rat’s formalin test. Pharmacologyonline 2008, 3, 659-664.
- Karabay-Yavasoglu, N.; Karamenderes, C.; Baykan, S.; Apaydin, S., Antinociceptive and Anti-inflammatory Activities and Acute Toxicity of Achillea nobilis. subsp. neilreichii. Extract in Mice and Rats. Pharmaceutical biology 2007, 45 (2), 162-168.
- Pires, J. M.; Mendes, F. R.; Negri, G.; Duarte-Almeida, J. M.; Carlini, E. A., Antinociceptive peripheral effect of Achillea millefolium L. and Artemisia vulgaris L.: both plants known popularly by brand names of analgesic drugs. Phytotherapy research : PTR 2009, 23 (2), 212-9.
- Abdel-Rahman, R.; Alqasoumi, S.; El-Desoky, A.; Soliman, G.; Paré, P.; Hegazy, M.-E., Evaluation of the anti-inflammatory, analgesic and anti-ulcerogenic potentials of Achillea fragrantissima (Forssk.). South African Journal of Botany 2015, 98, 122-127.
- Prut, L.; Belzung, C., The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. European journal of pharmacology 2003, 463 (1-3), 3-33.
- Zimcikova, E.; Simko, J.; Karesova, I.; Kremlacek, J.; Malakova, J., Behavioral effects of antiepileptic drugs in rats: Are the effects on mood and behavior detectable in open-field test? Seizure 2017, 52, 35-40.
- Okuyama, E.; Umeyama, K.; Saito, Y.; Yamazaki, M.; Satake, M., Ascaridole as a pharmacologically active principle of “Paico,” a medicinal Peruvian plant. Chemical & pharmaceutical bulletin 1993, 41 (7), 1309-11.
- Radulovic, N. S.; Dekic, M. S.; Randelovic, P. J.; Stojanovic, N. M.; Zarubica, A. R.; Stojanovic-Radic, Z. Z., Toxic essential oils: anxiolytic, antinociceptive and antimicrobial properties of the yarrow Achillea umbellata Sibth. et Sm. (Asteraceae) volatiles. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 2012, 50 (6), 2016-26.
- Alves, R.; de Carvalho, J. G. B.; Benedito, M. A. C., High and low rearing subgroups of rats selected in the open field differ in the activity of K+-stimulated p-nitrophenylphosphatase in the hippocampus. Brain research 2005, 1058 (1-2), 178-182.
- Anzoise, M. L.; Marrassini, C.; Ferraro, G.; Gorzalczany, S., Hydroalcoholic extract of Urtica circularis: A neuropharmacological profile. Pharmaceutical biology 2013, 51 (10), 1236-1242.

This work is licensed under a Creative Commons Attribution 4.0 International License.









