Tyrosinase Inhibitory Effect, Antioxidant and Anticancer Activities of Bioactive Compounds in Ripe Hog Plum (Spondias Pinnata) Fruit Extracts
Supawadee Patathananone*1 , Jureerut Daduang2, Amonrat Koraneekij2 and Chia-Ying Li3
, Jureerut Daduang2, Amonrat Koraneekij2 and Chia-Ying Li3
1Department of Chemistry, Faculty of Science and Technology, Rajamangala University of Technology Thanyaburi, 39 Moo 1, Rangsit-Nakhonnayok Rd., Klong 6, Thanyaburi, Pathum Thani, 12110, Thailand.
2Department of Clinical Chemistry, Faculty of Associated Medical Sciences, Khon Kaen University, Khon Kaen 40002, Thailand.
3Department of Applied Chemistry, National Pingtung University. No.1, Linsen Road., Pingtung City, Pingtung Country 90003, Taiwan.
Corresponding Author E-mail: Supawadee_P@rmutt.ac.th
DOI : http://dx.doi.org/10.13005/ojc/350302
Article Received on : 12-04-2019
Article Accepted on : 13-05-2019
Article Published : 12 Jun 2019
The usage of ripe hog plum fruit (Spondias pinnata) extracts in cosmetics and food products, including cancer therapeutic agents, have a few studies. Herein, the strong anti-tyrosinase activity found in the extracted part of isopropanol is reported. This extract was separated by liquid/liquid extraction using hexane: methanol+H2O. The hydrophilic layer (6A*) exhibited the anti-tyrosinase, antioxidant, and anticancer activities in vitro. The IC50 value of each bioactivity was presented as approximately 0.18, 0.04, and 1.40 mg/ml, respectively. In addition, 6A* fraction showed a very low cytotoxic effect in normal fibroblast cells (NHDF cells). The bioactive agents in 6A* were purified by C18 reverse-phase High-Performance Liquid Column Chromatography (HPLC). The 12 purified peaks were shown in the chromatogram profile. All peaks (excepted 6A-06 and 6A-09) displayed anti-tyrosinase activity, whereas the antioxidant property was not found in 6A-01, 6A-06, and 6A-08 but was represented in other peaks. Most purified peaks were indicated to be the aromatic alcohol or derivative phenol compounds.
KEYWORDS:Cytotoxic Effect; Flavonoid; Phenolic Acid; Tyrosinase Inhibitor; Wild Mango
Download this article as:| Copy the following to cite this article: Patathananone S, Daduang J, Koraneekij A, Li C. Tyrosinase Inhibitory Effect, Antioxidant and Anticancer Activities of Bioactive Compounds in Ripe Hog Plum (Spondias Pinnata) Fruit Extracts. Orient J Chem 2019;35(3). |
| Copy the following to cite this URL: Patathananone S, Daduang J, Koraneekij A, Li C. Tyrosinase Inhibitory Effect, Antioxidant and Anticancer Activities of Bioactive Compounds in Ripe Hog Plum (Spondias Pinnata) Fruit Extracts. Orient J Chem 2019;35(3). Available from: https://bit.ly/2KgQXdE |
Introduction
Aging is a normal situation of human life associated with two major factors: (1) intrinsic (decreasing hormone, increasing age, etc.) and (2) extrinsic factors (sunlight, smoking, etc.). In particular, reactive oxygen species (ROS) production increases in cells associated with the ultraviolet radiation from sunlight. ROS is a group of free radicals that enhance the activity of elastase, collagenase, and hyaluronidase enzymes. These enzymes can degrade the structural components of extracellular matrix (elastin, collagen, hyaluronic acid) in cells, changing the physiological and progressive structure of the skin1 (Garg et al., 2017). Moreover, tyrosinase, which is the key enzyme in the melanogenesis process, can be induced directly by sunlight; excessive exposure to sunlight can promote hyperpigmentation of the skin2,3 (Nema et al., 2011; Piwowarski et al., 2011).
There are two goal targets for decreasing the causes of aging skin: (1) inhibiting the enzyme activity and (2) decreasing oxidative stress by antioxidant agents.1 Furthermore, the active elements should be safety, insufficient side effects, and non-toxicity4 (Thring et al., 2009). Plants have been long reported to be used in the dietary life of humans. Plant metabolites are synthesized for use in several survival mechanisms, including defending against attacks from predators5 (Sanni and Omotoyinbo, 2016).Phytochemicals are a large group of plant-derived substances that consist of two types of metabolites, primary and secondary metabolites6 (Hussain et al., 2017). The primary metabolites are found in all plants, whereas secondary metabolites are synthesized for specific functions such as reducing oxidative stress and protecting plants from infection7 (Kasoteet al., 2015). Interestingly, plant metabolites are used in the development of many products that depend on the bioactivities of each compound. In particular, traditional medicinal plants have been important sources for identifying the compounds that have potential to be drugs, including bioactive agents used as the active ingredients in food preservatives and cosmetic products8 (Li et al., 2014).
Hog plum, or wild mango, is the general name of the genus Spondias, which consists of 18 species and is classified as part of the Anacardiaceae family9 (Sameh et al., 2018). The members of this genus have been used comprehensively in traditional medicine to treat many diseases. The pharmaceutical properties of the members show antioxidant10 (Hazra et al., 2008), anti-inflammatory11 (Cabral et al., 2016), antimicrobial12 (Arif et al., 2016), anti-arthritic, antipyretic, antifertility, antihypertensive, anticancer, hepatoprotective, hypoglycemic, and photoprotective effects.9 The Spondias pinnata is a species of the genus Spondias.9 Its native origin area is in Southeast Asia, including Thailand. The common name of S. pinnata in Thai is Makok. Recently, S. pinnata was a famous plant used in traditional foods in the northeastern area of Thailand, especially papaya salad and spicy foods. It was also used as an ingredient in traditional medicine. Moreover, in India, powdered ripe fruits of S. pinnata were used as an antidote for poison arrows.9 Researchers have reported that S. pinnata was a source that contained different classes of secondary metabolites. The methanolic extract of S. pinnata bark was found to be methyl gallate, which is able to induce apoptotic cell death in human glioblastoma, lung, and breast cancer13 (Chaudhuriet al., 2015). Furthermore, the chloroform/methanol extracts of S. pinnata bark exhibited ergosteryl triterpenes I and II14 (Adhikari et al., 2014). The methanolic extract of the S. pinnata fruitcontained triterpenoid substances such as β-amyrin and oleanolic acid15 (Singh and Saxena, 1976). The aerial parts of S. pinnata extracted by ethanol were found to include many kinds of sterols (β-sitosterol, and β-sitosterol β-D-glucoside, lignoceric acid, and stigmast-4-en-3-one, 24-methylenecycloartanone).9 However, a few researchers studied the anti-tyrosinase, anti-elastase, and anticancer activity on oral carcinoma cells from ripe hog plum (S. pinnata) fruit extracts. Additionally, the applications of ripe hog plum fruit extracts in cosmetic and food preservative products have not been reported. Therefore, the aim of this study was to extract the bioactive compounds from ripe hog plum (S. pinnata) fruit and to determine the anti-tyrosinase and cytotoxic activity in oral carcinoma (KB cells), including the cytotoxic effects in normal fibroblast cells (NHDF cells). Moreover, the bioactive agents were separated and purified by C18 reverse-phase HPLC. The purified peaks were analyzed for anti-tyrosinase and antioxidant properties, including the possibility of phytochemical types and their structures, through thin-layer chromatography and 1H NMR.
This report indicated that ripe hog plum fruit is a source of bioactive components that display the ability of anti-tyrosinase, including antioxidant and anticancer activities. This report is the first to use ripe wild mango fruit to extract the bioactive agents, as well as the first report to find the anti-tyrosinase activity of these extracts. Interestingly, these compounds showed very low cytotoxic effect in NHDF cells. Most of the bioactive agents contained in this extract are part of the group of polyphenolic compounds. In particular, gallic acid and gallic glycoside are substances indicated in this extract part. Previously, reports showed that gallic acid is a safety agent used for food additives23 and as an anticancer agent32. Therefore, the safety of the agents in the 6A* fraction could be developed to be the active ingredients in food and cosmetic products, including cancer therapeutic agents, in the future.
Materials and Method
Chemicals and Reagents
Mushroom tyrosinase was bought from Sigma Aldrich. L-Dopa and kojic acid were purchased from Himedia company. Dimethyl sulfoxide, folin ciocalteu reagent, trypan blue, Dulbecco’s Modified Eagle Medium-high glucose (DMEM-Hg), fetal bovine serum (FBS), L-glutamine, penicillin-streptomycin, and trypsin–EDTA were ordered from Gibco BRL (Grand Island, NY, U.S.A). Additionally, 3-[4, 5-dimethylthiazol-2-yl]-2-5-diphenyltetrazolium bromide (MTT) and 2,2-diphenyl-1-picrylhydrazyl were bought from Invitrogen (Carlsbad, CA).
Extraction Procedure
Ripe hog plum fruits were collected from the northeastern area of Thailand. They were washed with water including 70% v/v ethanol 3 times. Then, the cleaned fruits were dried in the air. Next, peel and pericarp were collected and homogenized using a homogenization machine. The homogenate was frozen dry in a freeze dryer. Approximately 200.0 g of dried powder was placed into the chambers, and hexane was added at a ratio of 1:4 w/v. The extracted chambers were shaken at 180 rpm for 72 h. The supernatant was collected and filtrated by Whatman No. 1. The precipitant was extracted continuously using ethyl acetate, isopropanol, and ethanol, respectively, using the same process of the hexane extraction. The schematic diagram of the extraction step is shown in Figure 1. All supernatant parts of each extraction solvent were evaporated using an evaporator. The extracts were named WMRI_Hexane, WMRI_Ethylacetate, WMRI_Isopropanol, and WMRI_Ethanol, respectively. Then, the inhibitory effect of these treatment samples on tyrosinase enzyme was determined.
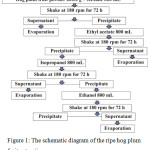 |
Figure 1: The schematic diagram of the ripe hog plum fruit extraction process. |
The extracts were named according to the solvent system used for the extraction procedure. The ID names of the extracts were WMRI_Hexane, WMRI_Ethylacetate, WMRI_Isopropanol, and WMRI_Ethanol.
Liquid/Liquid Extraction
The extract, WMRI_Isopropanol, was collected to extract the bioactive agents by liquid/liquid extraction16 (Punyatong et al., 2008). Approximately 10.0 g of this extract (WMRI_Isopropanol) was dissolved by hexane and placed into a separatory funnel. Next, methanol, which was added to a mini volume of the water, was subjected and mixed gently. The separatory funnel stood for 30 minutes. After that, the methanol layer was collected to evaporate and lyophilize. This part of the treated sample was called 6A* fraction. The biological properties and types of bioactive substances will be analyzed in the following procedure.
Anti-Tyrosinase Activity Assay
The inhibition tyrosinase activity effect was determined by the dopachrome method according to the modified procedure17 (Long et al., 2002). The reaction was conducted in a 96-well microplate. Kojic acid was used as a positive control. Firstly, the tyrosinase enzyme was pre-incubated with the varying concentrations of treatment samples. The 10.0 µg/ml of mushroom tyrosinase in 50 mM phosphate buffer pH 6.8 (100 µl) and 70 µl of deionized water were placed into wells of 96-well plate. Then, 10.0 µl of treatment samples were subjected and mixed with the enzyme. The plate was incubated at 37°C for 10 minutes. Next, 10.0 µl of 20.0 mM L-Dopa in 50 mM phosphate buffer pH 6.8 was added to the well and mixed gently. The plate was incubated at 37°C for 20 minutes. The absorbance value was measured at the wavelength of 495 nm of a microplate reader (EZ-Read 2000, Biochrome, U.S.A). The percentage of tyrosinase inhibitory activity of test substances was calculated according to the method described by Long et al.(2002).
Antioxidant Activity Assay
The 6A* fraction and the purified peaks were determined the antioxidant activity using a modified DPPH assay. The conditions of the analysis were conducted in a 96-well plate. Approximately 190 µl of the 0.10 mM DPPH solution was placed into each well. After that, 10 µl of the different concentrations of treatment samples and DMSO, which was used as the negative control, were added to the DPPH solution and mixed gently. The plate was incubated at 37°C for 30 minutes in the dark. Next, the absorbance value of each condition was measured at the wavelength of 515 nm using a microplate reader. The percentage of antioxidant activity was numbered using the equation previously described by Chen et al. (2010).18
The Cytotoxicity Assay
The cell viability assay most commonly used throughout the world is the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide or MTT assay previously described by Mossmann (1983).19 Herein, the epidermal carcinoma of the mouth (KB cells) and NHDF cells were placed in 25 cm2 tissue culture flasks at 37°C with 5% CO2 in DMEM-HG media supplemented with 10% fetal bovine serum and 1.0% penicillin-streptomycin (10,000 U/ml penicillin and 10 mg/ml streptomycin). Once the cells were approximately 70% confluent, they were trypsinized with 1 ml of 1X trypsin-EDTA, incubated at 37°C for 5 minutes, and centrifuged at 1,500 rpm for 5 minutes. The supernatant was removed, and 200 µl of seeding cells were re-suspended in 2 ml of DMEM-HG media. Approximately 9×103 cells were seeded in each well of the 96-well tissue culture plate and incubated under the same conditions for 24 h. Next, cells were treated with various final concentrations of 6A* (0.5–2 mg/ml) and incubated at 37°C for 24 and 48 h. After that, 100 µl of 5.0 mg/ml MTT solution in 1X PBS pH 7.4 was added to the wells and incubated for 4 h. Then, the MTT solution was removed. The solubilizing solution of formazan was subjected in each well and incubated at room temperature or 37°C. The dissolved formazan was measured for absorbance at 570 nm. The percentage of cells viability was calculated according to the formula (see formula below)as described by Patathananone et al. (2016).20
![]()
Thin Layer Chromatography
The treatment samples of 6A* and gallic acid were dissolved in methanol. Both samples were spotted on a silica sheet. Next, the mobile phase (chloroform: methanol: H2O; 1:1:0.0005) was placed into the chamber and incubated for 5 minutes. The stationary phase was put in the chamber and separated until the finish, and then it was incubated in the oven at 105°C for 10 min. The process of development was designed under the condition of KMnO4, 12% v/v H2SO4, and 0.05% v/v Anisaldehyde in acid methanol. The separated sheets were sprayed with each developing solution. Finally, separated TLC sheets were placed in an oven at 105 °C for 10 minutes to promote the spotting of the compounds.
Purification Active Bioactive Compounds by C18 Reverse Phase High Pressure Liquid Column Chromatography
The treatment sample 6A* was dissolved in 30% v/v methanol in deionized water and filtrated using a 0.45-µm syringe filter membrane. The column was incubated in 30% v/v methanol for 15 minutes at a flow rate of 3.0 ml/minutes. The 500 µl of the sample was injected into the C18 reverse-phase column (VP250/10 Nucleodur C18 HTEC, 5 µm) and separated by HPLC (SHIMADZU, Model SPD-20A) under the time program shown in Table 1. Absorbance values of each peak were determined at 220 nm and 270 nm. Next, the purified peaks were collected and evaporated to determine tyrosinase inhibitory effect and 11H NMR in the next procedure.
Determination Type of Phytochemicals, Including the Aromatic Alcohol and Derivative Phenol
The phenolic component in these purified peaks was analyzed by modified total phenolic content method21 (Dewanto et al., 2002). The experiment was set in a small volume of the reagent and showed the changing result on the silica sheet. Gallic acid and quercetin were used as the positive of phenolic and flavonoid standard compound, respectively. On the other hand, methanol was subjected to indicating as the negative control. Purified samples and control agents (10.0 µl) were placed into each well of 96-well plate. Then, 10.0 µl of 2.0% w/v Na2CO3 was added into wells. After that, 10.0 µl of diluted folin reagent was subjected and mixed. The plate was incubated in the dark for 20 minutes. Approximately 5.0 µl of each reaction was dropped on a silica sheet and dried at room temperature. The phenolic compounds were indicated by the blue-black, blue-green, and green color associated with the concentration of the agents.
Determination the Structure of Purified 6A-03 Peak by Proton Nuclear Magnetic Resonance
1H NMR spectra were recorded on JNM-CRZ 400 MHz spectrometers using tetramethylsilane (TMS) as the internal standard. The sample was dissolved in 0.6 mL DMSO-d6 for analysis. All chemical shifts are reported in parts per million (ppm, d ).
Statistical Analysis
The data of anti-tyrosinase activity and the cytotoxic activity assay were examined and carried out in triplicate. Statistical analysis of variance (ANOVA) was applied to evaluate the data.
Results
Inhibition Tyrosinase Activity of Crude Extracts
Ripe wild mango powder was extracted using the solvent system: hexane, ethyl acetate, isopropanol, and ethanol. These extracts were called WMRI_Hexane, WMRA_Ethyl acetate, WMRI_Isopropanol, and WMRI_Ethanol, respectively. The extracts were treated at 0.2-2.0 mg/ml. These results are presented in Figure 2. All treatment samples showed tyrosinase inhibitory activity. The percentage of tyrosinase inhibition of WMRI_Hexane and WMRA_Ethyl acetate was less than 40%. Both extracts might have an IC50 value higher than 2.0 mg/ml. Additionally, WMRI_Isopropanol extract presented strong anti-tyrosinase activity. The percentage of tyrosinase inhibition was higher than 70% at 2.0 mg/ml of test concentration. An IC50 dose of WMRI_Isopropanol was approximately 1.50 mg/ml. In the case of WMRI_Ethanol extract, the percentage of tyrosinase inhibition was higher than 40% at 1.0 mg/ml of treatment concentration. However, in this extract, we were unable to detect the IC50 value. The potential of tyrosinase inhibitory effect was suggested in WMRI_Isopropanol; leading to these extracts were collected to examine the biological properties and bioactive compounds in the next experiments.
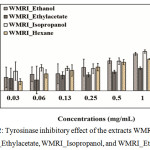 |
Figure 2: Tyrosinase inhibitory effect of the extracts WMRI_Hexane, WMRI_Ethylacetate, WMRI_Isopropanol, and WMRI_Ethanol. |
The results represent the percentage of inhibition indicated by mean ± SD (n = 3).
Anti-Tyrosinase Activity of 6A* Fraction
WMRI_Isopropanol was divided into two layers: (1) non-polar layer and (2) polar layer, called 6A*. Both extracted parts inhibited tyrosinase activity in vitro. The non-polar layer showed than 40% tyrosinase inhibition (data not shown). The IC50 dose of the non-polar layer might have been higher than 0.50 mg/ml. In addition, the high polarity substances in the 6A* fraction displayed vigorous anti-tyrosinase activity. The data are exhibited in Figure 3. The percentage of tyrosinase inhibition was increased to 80% after being treated with 0.40 mg/ml of this extracted part. The IC50 value represented approximately 0.18 mg/ml. This result indicated that the bioactive agents in 6A* displayed dose-dependent anti-tyrosinase activity.
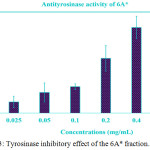 |
Figure 3: Tyrosinase inhibitory effect of the 6A* fraction. |
The different concentrations of this sample were treated with mushroom tyrosinase. The data of the treatment test that presented concentrations of 0.2, 0.4, and 0.5 mg/ml. We were shown to be statistically significant compared to the control (P < 0.05). The IC50 value was indicated to be approximately 0.18 mg/ml.
Antioxidation Activity of 6A* Fraction
DPPH is the free radical compound that has been extensively used to assay the free radical scavenging ability of manifold specimens. The antioxidant agents are able to decrease free radicals by donation of the electron (H atom) to the DPPH radical. The percentage of antioxidant activity is exhibited in Figure 4. The result indicated that phytochemical compounds in 6A* displayed scavenging activity with an IC50 dose of 48.0 µg/ml. The increasing concentration of this sample promoted the antioxidant ability in vitro. Approximately 80% of the inhibitory activity was indicated at a test concentration higher than 50 µg/ml.
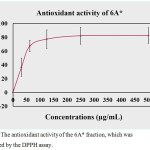 |
Figure 4: The antioxidant activity of the 6A* fraction, which was determined by the DPPH assay. |
The Cytotoxic Effect of 6A* in the Oral Carcinoma Cell Line (KB Cells) and Normal Human Dermal Fibroblasts Cell Lines (NHDF Cells)
The oral carcinoma cell lines (KB cells) were treated with different concentrations of the 6A* fraction. The percentage of viability of KB cells is indicated in Figure 5A. The treatment sample (6A*) showed the cytotoxic effect in KB cells to be dose- and time-dependent. The IC50 value presented approximately 1.4 mg/ml of 6A*. Interestingly, the effect of 6A* in NHDF cells exhibited an IC50 dose higher than in KB cells (Figure 5B). This result indicated that the compounds in 6A* also showed very low cytotoxic effect in normal cells.
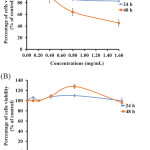 |
Figure 5: The cytotoxic effect of 6A* on KB cells (5A) and NHDF cells (5B). |
Both cell lines were treated with different concentrations of 6A* and incubated for 24 and 48 h, respectively. The concentrations of 0.8 and 1.6 mg/ml, incubated for 48 h, presented significance in terms of the percentage cell viability compared to the control (P < 0.05) and between groups of cell lines (KB cells and NHDF cells).
Thin Layer Chromatography of 6A*
The 6A* fraction that used the high polarity condition of the mobile phase was separated. The result is presented in Figure 6(A-C). The result presented in Figure 6(A) indicated that a spot of 6A* showed a similar Rf value to standard gallic acid, which is associated with the results in Figure 6(B) and 6(C). Moreover, the substance in 6A* that appeared as a green color (Figure 6[B]) might be glycoside or glycoside derivative. Thus, 6A* was purified by C18 reverse-phase HPLC to analyze the type of phytochemical compounds.
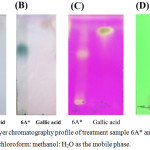 |
Figure 6: Thin-layer chromatography profile of treatment sample 6A* and gallic acid, performed using chloroform: methanol: H2O as the mobile phase. |
(A), (B), and (C) were developed by 12% v/v H2SO4, 0.05% Anisaldehyde in acid and KMnO4, respectively. Figure 6(D) shows the separation of 6A-03 (Lane 1) and standard gallic acid (Lane 2), for which the localization of the compounds was detected by ultraviolet light (254 nm).
HPLC Purification Profile of 6A*
The chromatography profile of purified 6A* by C18 reverse-phase HPLC is shown in Figure 7. The 12 purified peaks were collected: 6A-01, 6A-02, 6A-03, 6A-04, 6A-05, 6A-06, 6A-07, 6A-08, 6A-09, 6A-10, 6A-11, and 6A-12. All purified peaks were determined to exhibit tyrosinase inhibitory effect and antioxidant activity.
 |
Figure 7: Elution profile of purification treatment sample 6A* by C18 reverse-phase high-pressure column chromatography (HPLC). |
Time program of separation is shown in Table 1. The absorbance value was measured at 220 nm and 270 nm.
Table 1: The time program of treatment sample 6A* purification by C18 reverse-phase HPLC.
|
Times (min) |
Solvent system (%) |
Processing |
|
15 |
30% v/v solvent B |
equilibration |
|
Load /Injection sample |
||
|
0-30 |
30% v/v®50%v/v solvent B |
|
The Biological Properties of the Purified Peaks
The 12 purified peaks were analyzed for tyrosinase inhibitory activity. The data are shown in Table 2. There are 10 purified peaks able to inhibit tyrosinase activity in vitro: 6A-01, 6A-02, 6A-03, 6A-04, 6A-05, 6A-07, 6A-08, 6A-10, 6A-11, and 6A-12. The percentage of inhibition of each peak was higher than 70%; in particular, 6A-03 showed strong anti-tyrosinase activity, which 103% inhibition. Furthermore, the antioxidant activity was also represented in the 6A-02, 6A-03, 6A-04, 6A-05, 6A-07, 6A-09, 6A-10, 6A-11, and 6A-12 peaks. However, this bioactivity was not detected in the 6A-01, 6A-06, and 6A-08 peaks. This result is presented in Table 3. Interestingly, approximately 80% of antioxidant activity was shown in 6A-03 and 6A-09.
Table 2: The tyrosinase inhibitory effect of the purified peaks, including the percentage of tyrosinase inhibition.
| Samples | Tyrosinase inhibitory effect | Percentage of inhibition (% of control) |
| 6A-01 | + | 99 |
| 6A-02 | + | 99 |
| 6A-03 | + | 104 |
| 6A-04 | + | 98 |
| 6A-05 | + | 76 |
| 6A-06 | – | -8 |
| 6A-07 | + | 100 |
| 6A-08 | + | 72 |
| 6A-09 | – | -74 |
| 6A-10 | + | 100 |
| 6A-11 | + | 99 |
| 6A-12 | + | 96 |
| Kojic acid | + | 90 |
The symbols “+” and “-” mean the bioactive agents of the purified peak displayed anti-tyrosinase activity or the absence of tyrosinase inhibitory property, respectively.
Table 3: The antioxidant activity of purified peaks.
| Samples | Assayed Concentration | Antioxidant activity | Percentage of antioxidant activity (%) |
| 6A-01 | 100 µg/ml | ND | ND |
| 6A-02 | 100 µg/ml | Present | 47 |
| 6A-03 | 46 µg/ml | Present | 80 |
| 6A-04 | 28 µg/ml | Present | 33 |
| 6A-05 | 111 µg/ml | Present | 65 |
| 6A-06 | 43 µg/ml | ND | ND |
| 6A-07 | 60 µg/ml | Present | 69 |
| 6A-08 | 29 µg/ml | ND | ND |
| 6A-09 | 383 µg/ml | Present | 80 |
| 6A-10 | 106 µg/ml | Present | 74 |
| 6A-11 | 113 µg/ml | Present | 76 |
| 6A-12 | 169 µg/ml | Present | 72 |
Type of Phytochemical Compounds of Purified Peaks
Folin reagent was used to determine the phenolic compound in all purified peaks. A positive result was indicated by the blue-black/blue-blue light color, which is associated with the dose of the phenolic compound. The result is shown in Figure 8. Blue-black color was represented in gallic acid (positive control), 6A-09 and 6A-12, while 6A-03, 6A-04, 6A-05, 6A-07, 6A-10, 6A-11 exhibited blue color. In addition, 6A-01 and 6A-02 presented blue light color. However, the purified 6A-06 and 6A-08 presented a similar color spot with negative control (methanol). These results indicated that most phytochemical agents in 6A* contained aromatic alcohol in their structures. Therefore, phenolic compounds might be the major bioactive agents in the 6A* fraction. Furthermore, strong inhibitory tyrosinase effect and antioxidant activity appeared in 6A-03. Therefore, this purified peak was studied the structure of the bioactive agent using proton nuclear magnetic resonance spectroscopy (1H NMR). The 1H-NMR spectrum of 6A-03 showed only one signal at d 6.91 for the aromatic proton, which indicated that 1, 3, 4, 5-tetra substituted benzene ring moiety. The signals at d 8.80 (1H, OH) and 9.16 (2H, OH) indicated three phenolic alcohols. These data are very close to those of gallic acid in the literature22 (Xu et al., 2018). In addition, the separation profile of purified peak 6A-03 by TLC exhibited the Rf value similar to that of standard gallic acid. The result is shown in Figure 6(D).
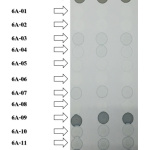 |
Figure 8: Determination of phenolic alcohol of purified peaks using folin reagent. |
The data are presented on a silica sheet. Blue-black and blue, including blue light color, represent the phenol ring or phenolic alcohol in their structure.
Discussion
Safety is an important target for developing health care products, especially cosmetics and food, including chemotherapeutic agents. In this study, plant extracts or natural compounds have been promoted for use as the active ingredients in various industrial products23 (Kim and Uyama, 2005). However, the lack of application of phytochemical agents from ripe wild mango (S. pinnata) fruit in cosmetics, food products, and cancer prevention encouraged us to implement this study.
Hyperpigmentation is a problem of the skin related to the melanogenesis process24 (Popoola et al., 2015) and associated with the catalytic activity of tyrosinase enzyme. Thus, the suppression of tyrosinase activity is a goal to decrease the melanin production of cells. According to previous reports, many natural products and synthetic compounds are used for subjection as the active components in whitening products1,23 (Garg et al., 2017; Kim and Uyama, 2005). However, high concentration of some anti-tyrosinase substances also showed side effects due to limitations in the application used. In this work, we indicated that the bioactive components from the extracted part of ripe wild mango fruit showed potential tyrosinase inhibitory effect, as well as antioxidant and anticancer activities. The polyphenolic/phenolic compounds are the major phytochemical substances found in this report.
Polyphenolic compounds are the major group of secondary metabolites in plants25 (Pedrosa et al., 2016). There are many kinds of phenolic and flavonoid compounds that have indicated the ability to exhibit tyrosinase inhibitory effects, as well as antioxidant and anticancer activities23,26,27,28 (Kim and Uyama, 2005; Kanadaswami et al., 2005; Kim, 2007; George et al., 2017). Our data showed that gallic acid is a phenolic compound represented in the 6A* fraction and purified peak 6A-03. It has been found in many kinds of plants, including the genus Spondias9 (Sameh et al., 2018). It exhibits many strong bioactivities: for example, antimicrobial, antioxidant, anticancer, and anti-inflammatory activities. Moreover, gallic acid also showed tyrosinase inhibitory activity. It displayed as a noncompetitive inhibitor in mushroom tyrosinase29 (Gheibi et al., 2016). Therefore, the tyrosinase inhibitory effect of 6A* and 6A-03 also participated in the presentation of gallic acid, which is related to previous reports. However, the phytochemical compounds of other purified peaks that showed anti-tyrosinase activity will be analyzed in future work.
Additionally, phenolics and flavonoids displayed a central role in antioxidant activity27 (Kim, 2007). Recent reports have indicated that ROS can enhance oxidative damage due to the disordered biomolecules in cells. Moreover, they were able to activate the enzyme’s activity of elastase, hyaluronidase, collagenase, which digested biomolecules (elastin, hyaluronic acid, and collagen) in the extracellular matrix of cells, leading to the wrinkled appearance of the skin1 (Garg et al., 2017). Furthermore, ROS can promote the activity of tyrosinase. This enzyme activated the melanogenesis process, which causes hyperpigmentation or melasma. Therefore, antioxidant agents play a key role in biomolecular disorder and aging prevention. The mechanism of antioxidation of phenolics and flavonoids has been explained by the fact that these compounds use the hydroxyl group in their benzene ring structure to donate an electron (H-atom) to free radicals30 (Pietta, 2000) due to the biomolecules in cells being protected from destruction by free radicals. Therefore, the strong antioxidant activity reported in our work might relate to the major polyphenolic agents in 6A*.
In food fields, tyrosinase is an enzyme that catalyzes the browning reactions in food products due to the nutritional quality being lost in storage. Thus, the methods that enable stopping the activity of tyrosinase enzyme are interesting and identified. Determining capable compounds is a method used to interrupt tyrosinase activity. However, the inhibition of enzymatic browning by chemical compounds has limited application in the food system. According to previous reports, sulfiting substances have been used for suppression of the enzymatic browning of seafood and agricultural products. These agents can be used in some food products, controlled by the Food and Drug Administration (FDA). Thus, food industries still continually determine the enzymatic browning inhibitors from natural sources to increase safety and avoid side effects. In our work, the phytochemical compounds that showed anti-tyrosinase activity and presented very low cytotoxicity with normal cells could be developed for use as food additives for inhibition of enzymatic browning of food products in the future23 (Kim and Uyama, 2005).
Recent reports have indicated that most chemotherapeutic compounds also exhibited side effects with normal cells, leading to unsatisfactory cancer treatments. To decrease the side effect of chemotherapeutic therapy, new anticancer agents have been identified from many sources31,32 (Dharap et al., 2005; Lin et al., 2007). The selective property of bioactive compounds plays a key role in decreasing side effects of cancer treatments in normal cells33 (Allen et al., 2004). Our report suggested that the bioactive compounds in 6A* showed very low cytotoxic effect in NHDF cells. These substances might be able to be used in discriminating between cancer cells and normal cells. Interestingly, polyphenolic compounds are the main group found in 6A*, including gallic acid. Previous studies have indicated that gallic acid exhibited anticancer activity in many types of cancer cells. Additionally, it presented no cytotoxic effect with primary cultured hepatocytes and macrophages of rats23,34 (Kim and Uyama, 2005; Sun et al., 2016). These results indicate that gallic acid is a phenolic compound that shows a selective property. Thus, gallic acid is studied widely for development as a cancer therapeutic agent. In 2015, Chaudhuri and co-workers reported on gallic acid and methyl gallate isolated from the ethyl acetate extraction of S. pinnata’s bark. Both agents showed a cytotoxic effect on human glioblastoma cell line (U87).13 Moreover, gallic acid can enhance G0/G1 phase arrest and apoptosis in human leukemia HL-60 cells by inhibiting cyclin D and E and activating the mitochondria-dependent pathway35 (Yeh et al., 2011). Herein, it is possible that the cytotoxic effect in KB cells may be associated with the pharmacological property of gallic acid. However, the anticancer activity of other phytochemical agents in our work will be analyzed in future work.
Overall, it can be concluded that ripe wild mango fruit is a source of bioactive compounds that display the tyrosinase inhibitory effect, as well as antioxidant and anticancer properties. Importantly, these compounds exhibited very low cytotoxicity with NHDF cells. Therefore, these phytochemical agents could be developed for an application used in cosmetic products, food additives, or cancer therapy in the future.
Acknowledgements
We would like to express our deepest and most sincere gratitude to the National Research Council of Thailand (NRCT), which supported the scholarship for this research. Additionally, the Department of Chemistry, Faculty of Science and Technology, Rajamangala University of Technology Thanyaburi, Faculty of Dentistry Khon Kaen University, Department of Applied Chemistry, National Pingtung University accommodated the research facilities.
Conflict of Interest
We wish to confirm that there are no known conflicts of interest associated with this publication, and there has been no significant financial support for this work that could have influenced its outcome.
References
- Garg, C.; Khurana, P.; Garg, M. Molecular mechanisms of skin photoaging and plant inhibitors. Int. J. Green. Pharmacy. 2017, 11(2), S217-S232.
- Nema, T.; Chan, E.C.; Ho, P.C. Efficiency of a miniaturized silica monolithic cartridge in reducing matrix ions as demonstrated in the simultaneous extraction of morphine and codeine from urine samples for quantification with liquid chromatography-tandem mass spectrometry (LC-MS/MS). J. Mass. Spectrom. 2011, 46(9), 891-900.
- Piwowarski, J.P.; Kiss, A.K.; Kozłowska-Wojciechowska, M. Anti-hyaluronidase and anti-elastase activity screening of tannin-rich plant materials used in traditional Polish medicine for external treatment of diseases with inflammatory background. J. Ethnopharmacol. 2011, 137(1), 937-941.
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC. Complement. Altern. Med. 2009, 9(1), 27-11.
- Sanni, D.M.; Omotoyinbo, O.V. Phytochemical Screening, Tyrosinase inhibitory effects and kinetics of cam wood dye extracts. Adv. Biochem. 2016, 4(2), 16-20.
- Hussain, R.; Shaukat, Z.; Khan, M.; Saint, R.; Gregory, S.L. Phosphoenolpyruvate carboxykinase maintains glycolysis-driven growth in Drosophila tumors. Sci Rep. 2017, 7(1), 1-11.
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11(8), 982-991.
- Li, N.A.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and biological activities of natural polyphenols. Nutrients. 2014, 6, 6020-6047.
- Sameh, S.; Al-Sayed, E.; Labib, R.M.; Singab, A.N. Genus Spondias: A Phytochemical and pharmacological review. Evid Based Complement Alternat Med. 2018, (4), 1-13.
- Hazra, B.; Biswas, S.; Mandal, N. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC. Complement. Altern. Med. 2008, 8, 1-10.
- Cabral, M.; Cheng, X.; Singh, S.; Ivessa, A.S. Absence of non-histone protein complexes at natural chromosomal pause sites results in reduced replication pausing in aging yeast cells. Cell. Rep. 2016, 17(7), 1747-1754.
- Arif, M.; Hussain, N.; Yasmeen, A. Morphological and physiological response of cotton to exogenous application of growth regulators. Agr. Res. J. 2016, 4(8), 467-477.
- Chaudhuri, R.; Knoblauch, K.; Gariel, M.A.; Kennedy, H.; Wang, X.J. A large-scale circuit mechanism for hierarchical dynamical processing in the primate cortex. Neuron. 2015, 88(2), 419-431.
- Adhikari, T.; Kundu, S.; Meena, V.; Subba Rao, A. Utilization of nano rock phosphate by maize (Zea mays L.) crop in a vertisol of central India. Agr. Sci. Tech. 2014, 4, 384-394.
- Singh, R.B.; Saxena, V.K. Chemical investigation on Spondias mangifera. The Institute of Chemists (India). 1976, 48, 299.
- Punyatong, M.; Pongpiachan, P.; Pongpiachan, P.; Kaladee, D.; Mankhetkorn, S. Cytotoxicity of crude extract proanthocyanidin from purple glutinous rice bran (Oryza sativa L.) Kum Doi Saket compared with cyanidin 3-glucosideon X63 myeloma cancer cells line. Kasetsart. J. (Natural Science). 2008, 42(4), 676-681.
- Long, H.H.; Schmidt, D.D.; Baldwin, I.T. Native bacterial endophytes promote host growth in a species-specific manner; phytohormone manipulations do not result in common growth responses. PLoS. One. 2008, 3(7), 1-10.
- Chen, Y.; Lawless, C.; Gillespie, C.S.; Wu, J.; Boys, R.J.; Wilkinson, D. J. CaliBayes and BASIS: integrated tools for the calibration, simulation and storage of biological simulation models. Brief. Bioinform. 2010, 11(3), 278-289.
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983, 65 (1-2), 55-63.
- Patathananone, S.; Thammasirirak, S.; Daduang, J.; Chung, J. G.; Temsiripong, Y.; Daduang, S. Inhibition of HeLa cells metastasis by bioactive compounds in crocodile (Crocodylus siamensis) white blood cells extract. Environ. Toxicol. 2016, 31(11), 1329-1336.
- Dewanto, V.; Wu, X.; Adom, K. K.; Liu, R. H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agr. Food. Chem. 2002, 50(10), 3010-3014.
- Xu, L.; Gong, W.; Cusack, S. A.; Wu, H.; Loovers, H. M.; Zhang, H.; Perrett, S.; Jones, G. W. The β6/β7 region of the Hsp70 substrate-binding domain mediates heat-shock response and prion propagation. Cell. Mol. Life. Sci. 2018, 75(8), 1445-1459.
- Kim, Y. J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic source: structure, inhibition mechanism and prospective for the future. Cell. Mol. Life Sci. 2005, 62, 1707-1723.
- Popoola, O. K.; Marnewick, J. L.; Rautenbanch, F.; Ameer, F.; Iwuoha, E. I.; Hussein, A. A. Inhibition of oxidative stress and skin aging-related enzymes by prenylated chalcones and other flavonoids from Helichrysum teretifolium. Molecules, 2015, 20, 7143 – 7155.
- Pedrosa, T. D.; Barros, A. O.; Nogueira, J. R.; Fruet, A. C.; Rodrigues, I. C.; Calcagno D. Q.; Smith, M. A.; de Souza, T. P.; Barros, S. B.; de Vasconcellos, M. C.; Silva, F. M.; Koolen, H. H.; Maria-Engler, S. S.; Lima, E. S. Anti-wrinkle and anti-whitening effects of jucá (Libidibia ferrea Mart.) extracts. Arch. Dermatol. Res. 2016, 308(9), 643 -654.
- Kandaswami, C.; Lee, L. T.; Lee, P. P.; Hwang, J. J.; Ke, F. C.; Huang, Y. T.; Lee, M. T. The antitumor activities of flavonoids. In vivo, 2005, 19, 895 – 910.
- Kim, Y. J. Antimelanogenic and antioxidant properties of gallic acid. Biol. Oharm. Bull. 2007, 30, 1052-1055.
- George, V. C.; Dellaire, G.; Rupasinghe, H. P. V. Plant flavonoids in cancer chemoprevention: role in genome stability. J. Nutr. Biochem. 2017, 45, 1 – 14.
- Gheibi, N.; Hosseini Zavareh, S.; Rezaei Behbahani, G. R.; Haghbeen, K.; Sirati-sabet, M.; Ilghari, D.; Goodarzvand, K. Comprehensive kinetic and structural studies of different flavonoids inhibiting diphenolase activity of mushroom tyrosinase. Appl. Biochem. Microbiol. 2016, 52(3), 304 – 310.
- Pietta, P. G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035 – 1042.
- Dharap, S. S.; Wang, Y.; Chandna, P.; Khandare, J. J.; Qiu, B.; Gunaseelan, S.; Sinko, P. J.; Stein, S.; Farmanfarmaian, A.; Minko, T. Tumor-specific targeting of an anticancer drug delivery system by LHRH peptide. Proc. Natl. Acad. Sci. U S A. 2005, 102(36), 12962-12967.
- Lin, J. P.; Yang, J. S.; Chang, N. W.; Chiu, T. H.; Su, C. C.; Lu, K. W, Ho, Y. T.; Yeh, C. C.; Mei, D.; Lin, H. J.; Chung, J. G. GADD153 mediates berberine-induced apoptosis in human cervical cancer CaSki cells. Anticancer. Res. 2007, 27, 3379 – 3386.
- Allen, T. M.; Cullis, P. R. Drug delivery systems: entering the mainstream. Sci. 2004, 303, 1818 – 1822.
- Sun, G.; Zhang, S.; Xie, Y.; Zhang, Z.; Zhao, W. Gallic acid as a selective anticancer agent that induces apoptosis in SMMC-7721 human hepatocellular carcinoma cells. Oncol. Lett. 2016, 11(1), 150 – 158.
- Yeh, T.C.1.; Liu, H. L.; Chung, C. S.; Wu, N. Y.; Liu, Y. C.; Cheng, S. C. Splicing factor Cwc22 is required for the function of Prp2 and for the spliceosome to escape from a futile pathway. Mol. Cell. Biol. 2011, 31(1), 43-53.

This work is licensed under a Creative Commons Attribution 4.0 International License.









