Synthesis and Biological Activity Studies of Methyl-5-(Hydroxymethyl)-2-Furan Carboxylate and Derivatives
Weerachai Phutdhawong1, Siwaporn Inpang2, Thongchai Taechowisan3 and Waya S. Phutdhawong*2
1Department of Science, Faculty of Liberal Arts and Science, Kasetsart University, Kamphaeng Sean campus,Nakhon Pathom, 73140, Thailand.
2Department of Chemistry, Faculty of Science, Silpakorn University, Nakhon Pathom 73000, Thailand.
3Department of Microbiology, Faculty of Science, Silpakorn University, Nakhon Pathom 73000, Thailand.
Corresponding Author E-mail: waya.sengpracha@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350322
Article Received on : 11-03-2019
Article Accepted on : 08-05-2019
Article Published : 14 May 2019
Methyl-5-(hydroxymethyl)-2-furan carboxylate and derivatives were prepared from furfuryl alcohol and their biological activities were studied for cytotoxicity against cancer cell lines HeLa, HepG2 and Vero, and Gram (+) and Gram (-) bacteria. The amine derivative, (5-(((2-(1H-indol-3-yl)ethyl)amino)methyl) furan-2-yl)methyl acetate, was found to have the most potent biological activity with IC50 62.37 µg/mL against the HeLa cell line and MIC 250 µg/mL against the photogenic bacteria.
KEYWORDS:Antibacterial Activities; Anticancer Activities; Furans; Methyl-5-(Hydroxymethyl)-2-Furan Carboxylate; Tryptamine
Download this article as:| Copy the following to cite this article: Phutdhawong W, Inpang S, Taechowisan T, Phutdhawong W. S. Synthesis and Biological Activity Studies of Methyl-5-(Hydroxymethyl)-2-Furan Carboxylate and Derivatives. Orient J Chem 2019;35(3). |
| Copy the following to cite this URL: Phutdhawong W, Inpang S, Taechowisan T, Phutdhawong W. S. Synthesis and Biological Activity Studies of Methyl-5-(Hydroxymethyl)-2-Furan Carboxylate and Derivatives. Orient J Chem 2019;35(3). Available from: https://bit.ly/2VB207p |
Introduction
Infectious diseases have been serious public health problems in previous decades.1-2 As drug resistance has evolved against antimicrobial and antineoplastic agents, the search for novel and potent antibacterial agents is necessary.3 Furans are an important heterocyclic class that possess variety of bioactivities.4-8 The furan moiety is considered to be a common structural motif in many natural products, and many research groups have reported the synthesis of substituted furans for biological assay. Methyl-5-(hydroxymethyl) – 2-furan carboxylate (1) was first discovered in 2009 from Curvularia lunata and reported as a toxin from fungal caused curvularia leaf spot of maize.9-10 In 2014, Yu-Tang Tung and co-workers isolated methyl-5-(hydroxymethyl)-2-furan carboxylate (1) from Antrodia camphorate11 and found it to possess anti-inflammatory activities.12 Recently the extraction of bacteria Streptomyces sp.13 from Zingiber zerumbet (L.) Smith14 in Chantaburi province, Thailand was reported to contain methyl-5-(hydroxymethyl)-2-furan carboxylate (1) together with many other biological compounds.15 This compound has interesting antibacterial activity with MIC 1.00 µg/mL against Staphylococcus aureus ATCC25923. This observation prompted us to synthesize new chemical entities incorporating the active furan pharmacophores to evaluate their biological activities. We report the synthesis of 1-(hydroxymethyl) furan derivatives (2-3) (Figure 1) and in vitro biological activities to identify viable leads for further studies.
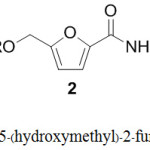 |
Figure 1: Structure of Methyl–5-(hydroxymethyl)-2–furan carboxylate 1 and derivatives. |
Materials and Methods
General Methods
Unless otherwise stated, nuclear magnetic resonance (NMR) was acquired in CDCl3 and on a Bruker Avance 300 spectrometer. Stuart Scientific SMP 2 was an apparatus used to measure the melting points and are uncorrected. Mass spectra were detected with a HEWLETT PACKARD 5973 or POLARIS Q mass spectrometer. To monitor the reactions, Merck silica plate 60 F254 was used for thin layer chromatography (TLC). Products were purified using Merck Kieselgel 60 via column chromatography.
Synthesis Method
General Procedure for the Synthesis of Amines Via Reductive Amination
A mixture of primary amine (1.0 equi), aldehyde (1.1 equi) and acetic acid (3 drops) in MeOH (10 mL) was heated under reflux for 1 h. After cooling to RT, sodium borohydride (0.6 equi) was added and stirred at 0°C for 1 h under Argon. The saturated NaHCO3 was added and extracted with EtOAc (3×20 mL). The organic phase was dried (Na2SO4), filtered and concentrated to give the dark brown residue. The residue was purified by flash column chromatography.
(5-(((2-(1H–indol–3–yl)ethyl)amino)methyl)furan–2–yl)methanol (8a)
Compound (8a) was obtained as yellow oil (30.40 mg, 10.5%) from tryptamine (0.10 g, 0.62 mmol) and 5-(hydroxymethyl)furan-2-carbaldehyde (5) (0.10 g, 0.62 mmol).; IR (ATR, cm-1) 3277, 3045, 2923, 1727, 1618, 1456, 1339, 1288, 1096, 1009, 795, 740, 583; UV (λmax, nm ) 203, 223, 281, 290; 1H NMR δ 2.94 (4H, t, J = 3.3 Hz, 2CH2), 3.70 (2H, s, NCH2), 4.46 (2H, s, OCH2), 6.06 (1H, d, J = 3.1 Hz, furan), 6.13 (1H, d, J = 3.1 Hz, furan), 6.93 (1H, d, J = 2.0 Hz, indole), 7.08 (1H, t, J = 7.5 Hz, CH-Ar), 7.17 (1H, t, J = 7.5 Hz, CH-Ar), 7.33 (1H, d, J = 8.0 Hz, CH-Ar), 7.57 (1H, d, J = 8.0 Hz, CH-Ar), 8.35 (1H, br, NH); 13C NMR δ 24.52, 45.14, 47.83, 56.41, 107.45, 107.48, 110.57, 118.03, 118.46, 121.22, 121.67, 126.59, 135.76, 152.27, 153.09, 153.51; HR-ESI MS calculated for C16H19N2O2 [M+H]+ 271.1447, found 271.1439.
(5-(((3–aminopropyl)amino)methyl)furan–2–yl)methanol (8b)
Compound (8b) was obtained as the unstable yellow oil (0.19 g, 25.6%) from the reaction of 1,3-diaminopropane (0.35 mL, 4.12 mmol and 5-(hydroxymethyl)furan-2-carbaldehyde (5) (0.49 g, 3.89 mmol). However, the colour of compound (8b) was changed from yellow to brown after left in the refrigerator without solvent for few days.);IR (ART, cm-1) 3270, 3050, 2925, 2851, 1628, 1595, 1555, 1456, 1339, 1227, 1096, 1009, 795, 740, 583; UV (λmax, nm ) 202, 223, 281, 290; 1H NMR δ 1.61 (2H, qui, J = 6.9 Hz, CH2-2′), 2.65 (2H, t, J = 6.9 Hz, CH2-1′), 2.73 (2H, t, J = 6.8 Hz, CH2-3′), 3.72 (2H, s, NCH2), 4.50 (2H, s, OCH2), 6.10 (1H, d, J = 3.0 Hz, furan), 6.16 (1H, d, J = 3.0 Hz, furan); 13C NMR δ 30.77, 37.87, 43.96, 44.49, 54.74, 105.44, 105.75, 151.36, 152.01; HR-ESI MS calculated for C9H17N2O2 [M+H]+ 185.1290, found 185.1283.
(5-(((2-(1H–indol–3–yl)ethyl)amino)methyl)furan–2–yl)methyl acetate (8c)
Compound (8c) was obtained as the yellow oil (50.57 mg, 13.5%)from (5-formylfuran-2-yl)methyl acetate (6) (0.20 g, 1.20 mmol) and tryptamine (0.29 g, 1.80 mmol).; IR (ATR, cm-1) 3275, 3056, 2920, 2850, 1734, 1619, 1456, 1339, 1230, 1095, 1009, 795, 740, 584; UV (λmax, nm ) 202, 222, 282, 290; 1H NMR δ 2.05 (3H, s, CH3), 2.98 (4H, s, 2CH2), 3.78 (2H, s, NCH2), 4.97 (2H, s, OCH2), 6.11 (1H, d, J = 3.2 Hz, furan), 6.30 (1H, d, J = 3.2 Hz, furan), 7.00 (1H, d, J = 2.2 Hz, indole), 7.10 (1H, t, J = 7.5 Hz, CH-Ar), 7.19 (1H, t, J = 7.5 Hz, CH-Ar), 7.36 (1H, d, J = 8.0 Hz, CH-Ar), 7.60 (1H, d, J = 7.8 Hz, CH-Ar); 13C NMR δ 19.12, 23.33, 43.83, 46.83, 56.45, 106.92, 109.72, 109.75, 110.75, 116.85, 117.33, 120.09, 120.81, 125.49, 134.85, 147.12, 151.76, 169.22; HR-ESI MS calculated for C18H21N2O3 [M+H]+ 313.1552, found 313.1543.
(5-(((3–aminopropyl)amino)methyl)furan–2–yl)methyl acetate (8d)
Compound (8d) (63.3 mg, 63.6%) was prepared from 1,3-diaminopropane (0.04 mL, 0.47 mmol) and (5-formylfuran-2-yl)methyl acetate (6) (73.8 mg, 0.44 mmol); (ATR, cm-1) 3273, 3050, 2924, 2850, 1627, 1593, 1554, 1456, 1339, 1227, 1095, 1009, 795, 740, 583; UV (λmax, nm ) 203, 222, 280, 290; 1H NMR δ 1.68 (2H, qui, J = 6.4 Hz, CH2–2′), 1.95 (3H, s, CH3), 2.69 (2H, t, J = 6.3 Hz, CH2-3′), 3.30-3.35 (2H, m, CH2-1′), 3.76 (2H, s, NCH2), 4.56 (2H, s, OCH2), 6.13 (1H, d, J = 3.0 Hz, furan), 6.21 (1H, d, J = 3.1 Hz, furan); 13C NMR δ 22.30, 28.14, 37.08, 45.21, 45.55, 56.34, 107.72, 108.15, 151.58, 153.75, 171.07; HR-ESI MS calculated for C11H19N2O3 [M+H]+ 227.1396, found 227.1394.
General procedure for the synthesis of amide derivatives (9a and 9b)
A mixture of methyl 5-(hydroxymethyl)-2-furan carboxylate (1) (1.0 equi), amine (1.0 equi) and triethylamine (1.0 equiv) in MeOH (10 mL) was refluxed overnight.The solvent was concentrated and purified by flash column chromatography.
N-(2-(1H–indol–3–yl)ethyl)-5-(hydroxymethyl)furan–2–carboxamide (9a)
Compound (9a) was obtained as the yellow oil of (88.80 mg, 28.1%) from methyl 5-(hydroxymethyl)-2-furan carboxylate (1) (0.20 g ,1.11 mmol) and tryptamine (0.18 g, 1.12 mmol); 1H NMR (CDCl3: MeOD, 9:1) δ 2.99 (2H, t, J = 6.9 Hz, CH2), 3.60-3.68 (2H, m, CH2), 4.48 (2H, s, OCH2), 6.26 (1H, d, J = 3.3 Hz, furan), 6.96 (1H, d, J = 3.3 Hz, furan), 6.98 (1H, s, indole), 7.08 (1H, t, J = 7.4 Hz, CH-Ar), 7.16 (1H, t, J = 7.4 Hz, CH-Ar), 7.35 (1H, d, J = 8.1 Hz, CH-Ar), 7.60 (1H, d, J = 7.7 Hz, CH-Ar); 13C NMR (CDCl3: MeOD, 9:1) δ 23.76, 38.28, 55.34, 108.14, 110.05, 113.53, 117.06, 117.69, 120.41, 121.04, 125.80, 135.08, 145.53, 155.05, 157.62, 157.69; HR-ESI MS calculated for C16H17N2O3 [M+H]+ 285.1239, found 285.1244.
N-(3–aminopropyl)-5-(hydroxymethyl)furan–2–carboxamide (9b)
Compound (9b) was obtained as the yellow oil of (0.14 g, 33.8%) from 1,3-diaminopropane (0.17 mL, 2.00 mmol) and methyl 5-(hydroxymethyl)-2-furan carboxylate (1) (0.32 g, 2.07 mmol); 1H NMR (MeOD) δ 1.77 (2H, qui, J = 6.8 Hz, CH2-2′), 2.72 (2H, t, J = 6.9 Hz, CH2-3′), 3.43 (2H, t, J = 6.9 Hz, CH2-1′), 4.59 (2H, s, OCH2), 6.47 (1H, d, J = 3.4 Hz, furan), 7.06 (1H, d, J = 3.4 Hz, furan); 13C NMR (MeOD) δ 31.76, 36.04, 38.21, 56.05, 108.96, 114.58, 146.94, 157.42, 159.65; HR-ESI MS calculated for C9H15N2O3 [M+H]+ 199.1083, found 199.1072.
(5-((2-(1H–indol–3–yl)ethyl)carbamoyl)furan–2–yl)methyl acetate (9c)
A mixture of 5-(Acetoxymethyl)furan-2-carboxylic acid (7) (0.13 g, 0.71 mmol) in DCM (5 mL), tryptamine (0.11 g, 0.67 mmol), dicyclohexyl carbodiimide (DCC) (0.21 g, 1.02 mmol) and 4-dimethylaminopyridine (DMAP) (8.26 mg, 0.07 mmol) was stirred for overnight. After that, the mixture was filtered and the filtrate was evaporated. The residue was purified by PLC (hexane: EtOAc, 2:1) to give yellow oil of (5-((2-(1H-indol-3-yl)ethyl)carbamoyl)furan-2-yl)methyl acetate (9c) 76.10 mg (34.6%).; 1H NMR (CDCl3: MeOD, 9:1) δ 2.06 (3H, s, CH3), 3.04 (2H, t, J = 6.9 Hz, CH2), 3.68-3.74 (2H, m, CH2), 4.98 (2H, s, OCH2), 6.44 (1H, d, J = 3.4 Hz, furan), 7.02 (1H, d, J = 3.6 Hz, furan), 7.03 (1H, s, indole), 7.09 (1H, t, J = 7.7 Hz, CH-Ar), 7.17 (1H, t, J = 7.7 Hz, CH-Ar), 7.38 (1H, d, J = 8.0 Hz, CH-Ar), 7.62 (1H, d, J = 7.8 Hz, CH-Ar); 13C NMR (CDCl3: MeOD, 9:1) δ 18.84, 23.45, 37.73, 55.85, 109.50, 110.85, 113.00, 116.65, 117.28, 120.02, 120.41, 125.38, 134.55, 134.70, 146.20, 149.22, 156.64, 168.85; HR-ESI MS calculated for C18H18N2NaO4 [M+Na]+ 349.1164, found 349.1158.
Biological Activity
Determination of Anticancer Activity
All the synthesised compounds were tested for cytotoxicity and anticancer activity by MTT assay on Vero cells (kidney of African green monkey cell line), HepG2 (human liver carcinoma cell line), and HeLa (human cervical carcinoma cell line).16 All cell lines were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum about 80% confluence. About 1 × 105cells/mL were plated on a 96-well microliter plates and incubated at 37°C under 5% CO2 for 24 h. The medium containing difference concentration of tested compounds was replaced. After 24 h of inoculation, 100 μL of the MTT solution (400 µg/mL) was added to each well and incubated at 37°C for 4 h, then the media were removed and added 100% DMSO 100 µL/well. Formazan was solubilized and the concentration was determined by optical density at 540 nm. The absorbance reading from each well was calculated the percentage of survival cells for each compound with different concentration.
Determination of Antibacterial Activity
Antibacterial activity was tested against four bacterial strains: B. subtilis ATCC 6633, B. cereus ATCC 7064, S. aureus ATCC 25932 and Escherichia coli ATCC10536 by disc diffusion method.17 The 500 μg of each compound were added in to a sterile paper disk. The disks were placed on nutrient agar plates that it containing of bacterial strains and were incubated at 37 °C for 24 h. The diameter zone of inhibition was measured in mm.
The minimal inhibitory concentration (MIC) was evaluated by micro broth dilution method (Taechowisan et al., 2018). The agents were dissolved in methanol (10,000 µg/mL) was serially diluted with nutrient broth supplemented with 10% glucose (NBG) and 0.5% phenol red as a pH indicator to get the varying concentration and 100 μL of each concentration was placed in the well. Then, 10 μL of the bacterial suspension (105 cells/mL) was inoculated into each well of a 96-well microplate. The drug-free and 5% methanol was used as a positive control and tetracycline was used as a reference. Microtiter plates were incubated for 24 h at 37 °C. MIC was defined as the lowest concentrations which no change of pH indicator.
Results and Discussion
Chemistry of Synthesis
The furfuryl alcohol (4) was converted to 5-(hydroxyethyl)-furaldehyde (5) by formylation using the Vilsmeier–Haack reaction according to Miyazawa et al.18 The o-acylation19 followed by Pinnick oxidation to afford carboxylic acid (7).20 Esterification of the carboxylic acid (7) under acidic conditions afforded the desired methyl-5-(hydroxymethyl)-2-furan carboxylate (1)21 (Scheme 1).
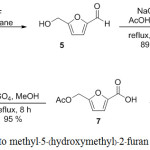 |
Scheme 1: Synthetic route to methyl–5-(hydroxymethyl)-2–furan carboxylate.Click here to view scheme |
The 5-(hydroxymethyl) furan derivatives were prepared by amination of aldehydes (5) and (6) with tryptamine and 1,3-diaminopropane. The amine derivatives (8a–8d) were obtained in low yields due to the solubility of the mixture in aqueous solution after worked up. The aminopropane derivative (8b) was unstable, the colour changed from yellow to brown after left in the refrigerator without solvent for few days. Thus, the fresh prepared compound (8b) was determined the structure by NMR spectroscopy and mass spectrum analysis prior to evaluate for the biological activities (Scheme 2).
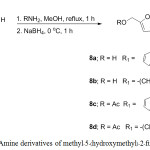 |
Scheme 2: Amine derivatives of methyl–5-(hydroxymethyl)-2–furan carboxylate.Click here to view scheme |
The acyl protection of alcohol compound (8c), compound (8d) was stable and the structure was compared with compound (8b) via NMR and mass spectrometry techniques and. To study the bio-activity of the amide derivatives of (9a-c), the reaction of methyl ester (1) with tryptamine and diaminopropane in the presence of the base triethylamine produced secondary amides (9a and 9b) in 28% and 34% yield respectively. Derivative (9c) was synthesized from the carboxylic acid (7) reacted with tryptamine via amide formation using dicyclohexylcarbodiimide (DCC) and dimethylaminopyridine (DMAP) to give 35% yield (Scheme 3).
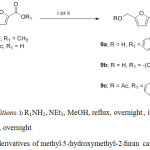 |
Scheme 3: Amide derivatives of methyl–5-(hydroxymethyl)-2–furan carboxylate. |
Biological Activity Assay
The cytotoxicity of the methyl-5-(hydroxymethyl)-2-furan carboxylate and derivatives was evaluated against HeLa, HepG2 and Vero cell lines using MTT assay. The IC50 are shown in Table 1. Compounds (1, 8c and 9c) showed weak cytotoxicity against the normal cell lines but significant anticancer activities against the HeLa cell lines. The presence of tryptamine and acyl ether in the structures led to an increase in anticancer activities, while the amide linkage reduced the activities in comparison with the amine linkage. In contrast, compound 8a showed selective anticancer activities against HepG2.
Table 1: IC50 of furan derivatives against the HeLa, HepG2 and Vero cell lines.
|
Compounds |
|
IC50(µg/mL) |
|
|||
|
HeLa |
HepG2 |
Vero |
||||
|
tryptamine |
275.26 |
149.08 |
193.64 |
|||
|
1 |
64.00 |
154.60 |
247.19 |
|||
|
8a |
143.64 |
96.98 |
137.45 |
|||
|
8b* |
215.01 |
150.03 |
307.39 |
|||
|
8c |
62.37 |
120.06 |
124.46 |
|||
|
8d |
200.73 |
135.90 |
241.67 |
|||
|
9a |
150.00 |
110.32 |
287.14 |
|||
|
9b |
270.03 |
157.00 |
204.76 |
|||
|
9c |
82.27 |
121.11 |
171.69 |
|||
* This compound was unstable and cytotoxicity was evaluated after preparation.
The synthesized compounds were also evaluated for antibacterial activities against pathogenic bacteria. The zones of inhibitions (mm) and MIC values are shown in Table 2. It was found that the synthetic compound (1) inhibited the Gram (+) bacteria; Staphylococcus aureus and Bacillus cereus with MIC 500.00 µg/mL, while all of the derivatives of (1) lost these activities. Thus, the methyl ester of compound (1) is necessary for inhibition. Most of the derivatives showed the ability to inhibit photogenic bacteria; B. subtilis and E. coli except the substituted aminopropanes (8b and 9b), which had no activities against any bacteria strains. Compound (8c) showed the most potent antibacterial activities against B. subtilis and E. coli (MIC 250.00 µg/mL) compared to all tested compounds. The amide derivative (9c) showed lower activity compared to (8c). This agrees with the importance of acyl and tryptamine substitution of the furan ring for anticancer activity.
Table 2: Antibacterial activity of furan derivatives against different bacteria.
|
Compounds |
B. cereus |
|
S. aureus |
B. subtilis |
E. coli |
|||||
|
ATCC7064 |
|
ATCC25923 |
ATCC6633 |
ATCC10536 |
||||||
|
IZ |
MIC |
IZ |
MIC |
|
IZ |
MIC |
IZ |
MIC |
||
| tryptamine |
– |
NT |
7.3 |
500 |
9.8 |
500 |
8.5 |
500 |
||
|
1 |
7.3 |
500 |
8.0 |
500 |
9.1 |
500 |
8.3 |
500 |
||
|
8a |
– |
NT |
– |
NT |
8.1 |
500 |
7.0 |
>500 |
||
|
8b |
– |
NT |
– |
NT |
– |
NT |
– |
NT |
||
|
8c |
– |
NT |
– |
NT |
19.0 |
250 |
10.0 |
250 |
||
|
8d |
– |
NT |
– |
NT |
6.9 |
>500 |
– |
NT |
||
|
9a |
– |
NT |
– |
NT |
13.3 |
500 |
8.0 |
500 |
||
|
9b |
– |
NT |
– |
NT |
– |
NT |
– |
NT |
||
|
9c |
– |
NT |
– |
NT |
9.0 |
500 |
8.0 |
500 |
||
|
tetracycline |
24.5 |
0.98 |
29.0 |
<0.49 |
27.3 |
0.98 |
25.3 |
3.91 |
||
IZ= zones of inhibitions (in mm), MIC= minimum inhibition (µg/mL)
(-) indicates = No zone of inhibition, NT = Not tested
All results are the average of triplicates.
Conclusion
Methyl-5-(hydroxymethyl)-2-furan carboxylate (5) and derivatives (8a-d), (9a-c) were prepared and tested for their biological activities. The core molecule (5) displayed both anticancer and antibacterial activity. The amine derivative, (8c) is more potent than the other compounds in this series, but it is not active against the bacteria S. aureus and B. cereus. Because of its carbonyl group, the amide derivative, (9c) is significantly less potent than (8c). Based on these results, the tryptamine and acyl protection of the primary alcohol are important for the furan ring to exhibit anticancer activity and antibacterial activity. These furan analogues could be served as important leads for further synthesis and optimization of the novel drug syntheses.
Acknowledgements
The authors are grateful to the Department of Chemistry and Department of Microbiology, Faculty of Science, Silpakorn University for the research support. We also gratefully acknowledged The Chulabhorn Research Institute for HRMS spectroscopy.
References
- Carlson, J. Scand. J. Public Health. 2006, 41, 132–138.
- Nii-Trebi, N.I. Biomed. Res. Int. 2017, 2017, 1–15.
- Shekhar, C. Cell Chem. Biol. 2010,17, 413–414.
- Mansouri, M.; Movahedian, A.; Rostami, M.; Fassihi, A. Res. Pharm. Sci. 2012, 7, 257–264.
- Dasagrandhi, C.; Ellamar, J. B.; Kim, Y. S.; Kim, H. R. Food Sci. Biotechnol. 2016, 25, 1671–1675.
- Chen, Y. L.; Zhao, Y. L.; Lu, C. M.; Tzeng, C. C.; Wang, J. P. Bioorg. Med. Chem. 2006, 14, 4373–4378.
- Schneider, K.; Keller, S.; Wolter, F. E.; Röglin, L.; Beil, W.; Seitz, O.; Nicholson, G.; Bruntner, C.; Riedlinger, J.; Fiedler, H. P.; Süssmuth, R. D. Angew. Chem. Int. Ed. 2008, 47, 3258 –3261.
- Matsuya, Y.; Sasaki, K.; Nagaoka, M.; Kakuda, H.; Toyooka, N.; Imanishi, N.; Ochiai, H.; Nemoto, H.. J. Org. Chem. 2004, 69, 7989–7993.
- Liu, T.; Liu, L.; Jiang, X.; Huang, X.; Chen, J. Can. J. Plant Pathol. 2009, 31, 22–27.
- Liu, T.; Liu, L.; Hou, J.; Li, G.; Gao, S.;Chen, J. Can. J. Plant Pathol. 2010, 32, 225–228.
- Geethangili, M.; Tzeng, Y.M. Evid. Based Complement. Alternat. Med. 2009, 2011, 1–17.
- Tung, Y.T.; Tsai, T. C.; Kuo, Y. H.; Yen, C. C.; Sun, J. Y.; Chang, W. H.; Chen, H. L.; Chen, C. M.. Phytomedicine. 2014, 21, 1708–1716.
- Hasani, A.; Kariminik, A.; Issazadeh, K. Int J Adv Biol Biom Res. 2014, 2, 63–75.
- Yob, N. J.; Jofrry, S. M.; Affandi, M.; The, L. K.; Salleh, M. Z.; Zakaria, A. Z. Evid. Based Complement. Alternat. Med. 2011, 2011,1–12.
- Puckdee W. PhD. Graduate School. Silpakorn University. Thailand. 2016.
- Taechowisan, T.; Chaisaeng,S.; Phutdhawong, W. S. Food Agric. Immunol. 2017, 28, 1330–1346.
- Taechowisan, T.; Jantiya, J.; Mungchukeatsakul, N.; Phutdhawong, W. S. Biomed J Sci & Tech Res. 2018, 3, 1–9.
- Miyazawa, M.; Anzai, J.; Fujioka, J.; Isikawa, Y. Nat. Prod. Res. 2003, 17, 337–339.
- Mehner, A.; Montero, A. L.; Martinez, R.; Spange, S. Molecules. 2007, 12, 634-640.
- Moore, J. A.; Partain, E. M. OPPI Briefs. 1985, 17, 203–205.
- Schmuck, C.; Machon, U. Eur. J. Org. Chem. 2006, 4385–4392.

This work is licensed under a Creative Commons Attribution 4.0 International License.









