Reactions of MoOCl4 with 1-Methylimidazole, 1,4-Diaminobutane, 2-Methylpyridine, 4-Methylpiperidine, Trimethylsilylimidazole and 1-Methylpyrrolidine
1Research Scholar Registered with Punjab Technical University, Kapurthala, 144603 - India.
2Department of Applied Chemistry, Giani Zail Singh Campus College of Engineering and Technology, Dabwali Road, MRSPTU Bathinda, 151001 - India.
Corresponding Author E-mail: gursharans82@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350324
Article Received on : 11-05-2019
Article Accepted on : 01-06-2019
Article Published : 20 Jun 2019
MoOCl4 reacts with 1-methylimidazole, 4-methylpiperidine and trimethylsilylimidazole (equimolar molar amounts) in solvent CH3CN to provide MoO2Cl4(C3H3N2CH3) [1], MoOCl4(C5H9NHCH3)CH3CN [4] and MoO2Cl2(C3H4N2), [7]. MoOCl4 reacts with twice the moles of 1,4-diaminobutane, 2-methylpyridine, 4-methylpiperidine and 1-methylpyrrolidine in solvent CH3CN to provide: MoOCl4(H2NC4H8NH2)2 [2], MoOCl4(C5H4NCH3), [3], MoOCl4(C5H9NHCH3)2, [5], MoOCl4(C5H9NHCH3)2, [6] and MoO2Cl4(C4H8NCH3)2, [8]. Complexes have been studied by techniques: elemental quantitative analysis, FTIR, 1H NMR, Mass (LC-MS).
KEYWORDS:1,4-Diaminobutane; MoOCl4; 1-Methylimidazole; 4-Methylpiperidine; 1-Methylpyridine; 1-Methylpyrrolidine; Trimethylsilylimidazole
Download this article as:| Copy the following to cite this article: Mangla V, Singh G. Reactions of MoOCl4 with 1-Methylimidazole, 1,4-Diaminobutane, 2-Methylpyridine, 4-Methylpiperidine, Trimethylsilylimidazole and 1-Methylpyrrolidine. Orient J Chem 2019;35(3). |
| Copy the following to cite this URL: Mangla V, Singh G. Reactions of MoOCl4 with 1-Methylimidazole, 1,4-Diaminobutane, 2-Methylpyridine, 4-Methylpiperidine, Trimethylsilylimidazole and 1-Methylpyrrolidine. Orient J Chem 2019;35(3). Available from: https://bit.ly/31Ear1n |
Introduction
Reactions of MoOCl4 with various ligands have been reported. Molybdenum in MoOCl4 being in VI oxidation state, it has the tendency to get reduced during reactions with ligands. Reactions may yield addition, substitution, reduction, rearrangement and polymerization products. Reactions of MoOCl4 in solvent CH2Cl2 have been reported1-5 by the author.Ligands are poorly soluble in solvent CH2Cl2, so reactions of MoOCl4 were also carried out and reported6-11 by the author in CH3CN medium.
Behavior of saturated N-heterocyclic ligands (4-methylpiperidine, 1-methylpyrrolidine) and unsaturated N-heterocyclic ligands (1-methylimidazole, trimethylsilylimidazole, 1-methylpyridine) towards MoOCl4 in solvent CH3CN at room temperature have been reported by the author in this paper.
FTIR, 1H NMR and Mass (LC-MS) spectra have helped in identifying the presence of the particular ligands in the compounds [1] to [8] synthesized. Further, Mass (LC-MS) spectra fragmentation pattern of these compounds supported their molecular formulae.
Aim of Investigation
Over the years, there has been increasing applications of Schiff bases and their transition metal complexes in biology, including antifungal, anti-inflammatory, antibacterial, anticancer, antimalarial, antiviral activity. Such studies on complexes of Schiff bases 1-methylimidazole, trimethylsilylimidazole; 1-methylpyridine with transition metals have been scarcely reported. Efforts have been done to prepare such compounds of molybdenum.
Alkylation increases the basic character of piperidine and pyrrolidine. Reactions of simple piperidine and pyrrolidine with MoOCl4 have already been reported1,2 by the author. Increased basicity of alkylated piperidine and pyrrolidine is expected to impact the substitution/addition and redox reactions with MoOCl4.
In view of the above, it was decided to carry out the current investigation.
Materials and Methods
Precursor MoOCl4 was synthesized in the lab. by refluxing SOCl2 with MoO3 (CDH, AR Grade) for 5 h. After the reaction, unreacted SOCl2 was removed with the help of vacuum and collected in in liquid nitrogen traps. Residue thus obtained was dark green in colour. It was treated with dry solvent CH2Cl2. Deep red solution was obtained. Red solution was passed through filtration unit having G-4 sintered glass crucible. MoOCl4 dark green crystals were obtained on evaporation of the filtrate.
Sigma-Aldrich ligands were used: 1-Methylimidazole (m.p./b.p. -6°C/124°C), 4-Methylpiperidine (m.p./b.p. 4° C/198°C), Trimethylsilylimidazole (m.p./b.p. -42°C/93°-94°C), 1,4-Diaminobutane (m.p./b.p. 25°-28°C/158°-160°C), 2-Methylpyridine (m.p./b.p. -70°C/128°-129°C), 1-Methylpyrrolidine (m.p./ b.p. -90°C/76°-80°C). These ligands were vacuum dried. LR grade thionyl chloride (b.p. 76°-78°C, CDH) was treated with quinoline for 2 days (250 g SOCl2 to 50 g quinoline) to eliminate acidic impurities. Fractional distillation was carried out to obtain colourless thionyl chloride. Solvent CH3CN was dried by standard procedure.
Mo and Cl were determined by standard procedures.12 C, H, N, O were determined with Thermo Finnigan Elemental Analyzer, 1H-NMR spectra have been recorded on Brucker Avance-II 400 (Fallanden) NMR in DMSO-d6, FTIR spectra in the range 4000 – 400 cm-1 were taken on Perkin-Elmer 400 FTIR Spectrometer (Germany), in KBr disks, LC-MS spectra were obtained in the range 0 – 1100 m/z using WATERS, Q-TOF Micromass LC-MS (UK), at Panjab University, Chandigarh (India), SAIF/CIL facility.
Preparation of Compounds1-8
One 100 ml rb flask was connected to a pressure dropping funnel fitted having teflon stop-cock. A magnetic bead was added in the flask. The whole unit was dried under vacuum flame drying. After cooling the unit, dry nitrogen gas was flushed into it. A known weight of MoOCl4 dissolved in dry CH3CN was taken in round bottomed flask. Equimolar or 1:2 molar amount of 1-methylimidazole, 4-methylpiperidine trimethylsilylimidazole, 1,4-diaminobutane, 2-methylpyridine, 4-methylpiperidine or 1-methylpyrrolidine were dissolved in solvent CH3CN and taken in dropping funnel. The ligand from the dropping funnel was mixed with MoOCl4 in rb flask at room temperature with constant stirring. Products were filtered through G-4 bed of a filtration unit, under reduced pressure. Compounds prepared are very much sensitive to air and moisture. They tend to turn blue in colour. All reactions were carried out under oxygen free dry nitrogen gas atmosphere in vacuum line. Liquid nitrogen cooled traps were used to get rid of moisture, oxygen.
There seems to be disproportionation/rearrangement during reactions. Filtrate or residue mentioned below the products, refers to the source from where the product has been isolated.
 |
Scheme 1 |
Results and Discussion
Analytical Measurements
Compounds are very much sensitive to moisture and air. They are insoluble in less polar solvents like n-hexane, CH2Cl2, CHCl3, but are soluble in solvents like CH3CN, DMSO and DMF of high polarity. These compounds have been formulated on basis of their elemental analysis and LC-MS studies (Table-I).
Table 1: Elemental Analysis.
| Compounds (Color/Formula Mass) | % Observed (Theoretical) | |||||
| Mo | Cl | C | H | N | O | |
| MoO2Cl2.(C3H3N2CH3)Cl2, [1](Green/352.0) | 27.72(27.27) | 40.92(40.34) | 14.12(13.63) | 02.27(01.70) | 08.11(07.95) | 08.72(09.09) |
| MoOCl4.(H2NC4H8NH2)2, [2](Light blue/434.0) | 21.23(22.12) | 32.23(32.72) | 21.19(22.12) | 06.28(06.45) | 12.34(12.90) | 03.82(03.68) |
| MoOCl4.(C5H4NCH3), [3](Black/347.0) | 26.95(27.66) | 40.45(40.92) | 20.23(20.74) | 02.17(02.02) | 04.74(04.03) | 04.57(04.61) |
| MoOCl4.(C5H9NHCH3)CH3CN, [4](Greyish blue/394.0) | 25.63(24.36) | 36.83(36.04) | 21.92(21.32) | 04.27(04.06) | 06.56(07.11) | 04.17(04.06) |
| MoOCl4.(C5H9NHCH3)2, [5](Dark brown/452.0) | 20.60(21.23) | 32.10(31.42) | 32.80(31.86) | 06.28(05.75) | 06.34(06.19) | 03.15(03.54) |
| MoOCl4.(C5H9NHCH3)2, [6](Greenish blue/452.0) | 21.70(21.23) | 30.90(31.42) | 17.81(31.86) | 05.74(05.75) | 05.85(06.19) | 03.13(03.54) |
| MoO2Cl2.(C3H4N2), [7](Parrot green/267.0) | 36.67(35.95) | 27.43(26.59) | 14.36(13.48) | 02.14(01.50) | 10.57(10.48) | 11.23(11.98) |
| MoO2Cl2.(C4H8NCH3)2Cl2, [8](Blue/440.0) | 22.33(21.82) | 33.10(33.27) | 27.72(27.27) | 05.15(05.00) | 05.95(06.36) | 06.93(07.27) |
FTIR Spectra
Close proximity of vibrational frequencies of 1-methylimidazole13,14 with that of [1] shows the presence of this ligand in [1]. Nitrogen at position 3 of 1-methylimidazole makes a coordinate bond with molybdenum. On Mo-N coordination, there is increase in ring C=C ring str. recorded at 1584.5 cm-1, 1548.9 cm-1. There is also increase in ring N-C str. observed at 1442.7 cm-1. This increase in frequencies is because of following 2 reasons:
Inductive effect due to coordination with positive metal ion,
dπ-pπ interactions dissipate the accumulation of negative charge.
This leads to increase in electron density in the ligand ring system. The greater the increase in ring frequency, the stronger is the Mo-N coordinate bond. Presence of cis-MoO22+ core15 in [1] is indicated by the presence of strong bands at 983.7 cm-1 and 918.1 cm-1 (Table-II).
Table 2: FTIR frequencies in cm−1.
| Mode | C3H3N2CH3 (1-Methylimidazole)13,14 | [1] |
| Ring C-H str. | 3015 m, 2953 w | 3291.8 vs, 3151.0 s |
| Ring C=C str. | 1517 vs | 1584.5 m, 1548.9 w |
| Ring N-C str. | 1407 m | 1442.7 m |
| C-H in plane bending | 1106 m, 1085 m, 1033 vw | 1155.7 w, 1084.9 w |
| C-H wagging, Ring twisting | 813 s, 772 s | 752.4 s |
| Ring twisting | 638 s | 622.5 |
| N-H wagging, Ring twisting | 523 | 571.5, 504.9 w |
| υ(Mo=O) of cis-MoO22+ core15 | —- | 983.7 vs, 918.1 w |
N-H stretching frequencies have been observed at 3413.1 cm-1, 3080.0 cm-1 and 3012.5 cm-1 in [2] (Table-III). A strong Mo=O stretching16,17 at 920.0 cm-1 shows the presence of terminal Mo=O. Bending mode due to NH2 observed in 1,4-diaminobutane18 at 1145 cm−1 is lowered to 1116.2 cm−1, because of Mo-N coordination.
Table 3: FTIR frequencies in cm−1.
| Mode | H2NC4H8NH2 (1, 4-Diaminobutane)18 | [2] |
| N-H Str. | 3346, 3280 | 3413.1 s, 3080.0 vs, 3012.5 vs |
| CH2 Str. | 2960-2875 | 2946.5 sh, 2881.5 sh |
| NH2 Bending | 1606 | 1612.7 m |
| CH2 Deformation (strong) | 1497, 1390, 1353, 1309 | 1470.8 m, 1446.5 s |
| NH2 Bending | 1145 | 1283.2 s, 1116.2 s |
| C-N sym str. (weak) | 1070 | 1028.3 m |
| CH2 Deformation (medium) | 863, 738 | 872.8 m, 765.0 w |
| Mo-N (Strong) | —- | 499.7 m |
| Terminal υ(Mo=O)16,17 | —- | 920.0 m |
Ring C-H stretching in 2-methylpyridine19-22 are obtained at 3137 cm-1 and 3066 cm-1. Ring C-H stretching in [3] are observed at higher frequencies 3296.0 cm-1 and 3081.0 cm-1. C=N Str. in [3] was observed at higher frequency at 1625 cm-1. C-N stretching in [3] was observed at lower frequency at 1288.3 cm-1 (Table-IV). All these observations indicate the presence of 2-methylpyridine in [3]. A strong Mo=O stretching at 980.0 cm-1 in [3] shows the presence of terminal Mo=O.16,17
Table 4: FTIR frequencies in cm−1.
| Mode | C5H4NCH3 (2-Methylpyridine)19-22 | [3] |
| Ring C-H Str. | 3137 m, 3086 m, 3066 m, 3012 s | 3296.0 s, 3081.0 s |
| Methyl C-H Str. | 2958 m | 2928.0 s, 2837.0 s |
| C=N Str. | 1596 vs | 1625.0 s |
| Ring C-C Str. | 1589 m | 1617.0 s |
| Ring C-H in plane bending | 1477 s | 1538.1 s |
| Methyl C-H Asym. bending | 1461 s | 1469.1 s |
| Methyl C-H Sym. bending | 1377 w | 1396.2 m |
| C-N Str. | 1295 s, | 1288.3 m |
| C-CH3 Str. | 1237 m | 1234.5 w |
| Ring C-H in plane bending | 1148 m, | 1165.2 s |
| Ring C-C Str. | 1101 w | 1108.5 w, 1096.6 w |
| Ring breathing | 1060 s | 1046.4 m |
| Ring C-H, C-C, C-N out of plane bending | 752 vs | 770.0 s |
| Ring C-H out of plane bending | 731 m | – |
| Ring C-C out of plane bending | 629 m | 627.1 w |
| Ring C-C-C in plane bending | 547 w | 566.3 |
| C-CH3 Bending | 471 | 471.3 s |
| Terminal Mo=O16,17 Str. | —- | 980.0 s |
There is N-H stretching at 3283 cm-1 in 4-methylpiperidine.23-26 Stretching at 3152.7 cm-1 in [4], 3088.7 cm-1 in [5] and 3088.7 cm-1 in [6] suggest presence of N-H group in these compounds. Decrease in frequency is due to coordination of N-H group through nitrogen atom in these compounds. Stretching at 980.8 cm-1, 975.8 cm-1 and 979.2 cm-1 in [4], [5] and [6], respectively, refer to Mo=O16, 17 group in terminal position (Table-V).
Table 5: FTIR frequencies in cm−1.
| Mode | C5H9NHCH3 (4-Methylpiperidine)23-26 | [4] | [5] | [6] |
| N-H Str. | 3283 m | 3367.2 s, 3152.7 s | 3377.1 sh, 3088.7 s | 3367.4 sh, 3150.0 s |
| CH3 Sym. Str. | 2967 s, 2915 s | 2957.7 s | 2953.0 s | 2956.1 s |
| Ring C-H Asym. Str. | 2800 s, 2732 m | 2808.1 w | 2847.4 s, 2791.5 s | 2806.6 sh |
| Ring C-H Deformation | 1456 m, 1448 m | 1610.7 s, 1452.7 m, 1407.3 w | 1609.0 sh, 1570.5 s, 1451.8 s | 1569.3 s, 1452.1 s |
| Ring C-C Str. | 1386 w, 1323 m | 1386.8 w, 1301.3 w | 1302.0 w | 1303.9 w |
| C-N Str. | 1265 w, 1153 m | 1223.9 w | 1224.7 w, 1178.9 w | 1223.5 w |
| Ring C-H Bending | 1007 w, 983 w, 972 w | 1065.9 w, 1038.0 w, 917.8 w | 1070.6 w, 1038.5 w, 954.9 m | 1068.9 w, 1038.1 w |
| CH2 Rocking | 795 m, 771 s | 762.4 s | 871.6 w, 722.1 s | 876.1 w, 839.1 w, 786.5 w, 724.1 m |
| CNC Deformation | 571 m | 568.2 m, 444.9 w | 569.8 w, 514.7 m, 447.9 w, 410.9 w | 567.8 w, 496.3 w, 445.0 w, 407.9 w |
| Terminal υ(Mo=O)16,17 | — | 980.8 s | 975.8 s | 979.2 s |
N-H stretching in imidazole27-29 are observed at 3724-3237 cm-1. [7] shows N-H broad stretching at 3246.0 cm-1 due to hydrogen bonding in the solid state (KBr disk). Close proximity of vibrational frequencies of imidazole27-29 with that of [7] shows the presence of this ligand in [7]. Nitrogen at position 3 of imidazole makes a coordinate bond with molybdenum. On Mo-N coordination, there is increase in ring C=C ring str. recorded at 1615.0 cm-1 and 1584.0 cm-1. There is also increase in ring N-C str. observed at 1436.6 cm-1. This increase in frequencies is because of the reasons already explained for [1]. Two bands attributable to the presence of stretching due to cis-MoO22+ core15 are observed at 972.9 cm-1 and 916.4 cm-1 in [7] (Table-VI).
Table 6: FTIR frequencies in cm−1.
| Mode | C3H4N2 (Imidazole)27-29 | [7] |
| υ(N-H) | 3724 b, 3656 b, 3270, 3241, 3237 | 3246.0 b |
| υ(C-H) | 3196, 3165 | 3149.1 s, 2993.1 sh |
| Ring υ(C=C) | 1558, 1500 | 1615.0 s, 1584.0 s, 1491.5 sh |
| Ring υ(N-C) | 1434 | 1436.6 m |
| δ(C-H) in plane | 1092, 1074 | 1094.0 w, 1069.7 m, 1048.9 w |
| δ(C-H) (wagging), Ring twisting | 816, 730 | 753.5 vs |
| Ring twisting | 646 | 643.5 sh, 621.3 w |
| Ring twisting, N-H wagging | 528 | 562.2 s |
| υ(Mo=O) of cis-MoO22+ core15 | —- | 972.9 s, 916.4 s |
1-Methylpyrrolidine30-32 has C-H symmetric stretching at 2973 cm-1 and C-H asymmetric stretching at 2892 cm-1, 2833 cm-1, 2782 cm-1. [8] has C-H symmetric stretching at 2971.5 cm-1 and C-H asymmetric stretching at 2727.6 cm-1. Two bands at are attributable to the presence of Stretching due to cis-MoO22+ core15 are observed at 983.7 cm-1 and 914.6 cm-1 in [8] (Table-VII).
Table 7: FTIR frequencies in cm−1.
| Mode | C4H8NCH3 (1-Methylpyrrolidine)30-32 | [8] |
| C-H Sym. Str. | 2973 s | 2971.5 s |
| C-H Asym. Str. | 2892 sh, 2833 m, 2782 s | 2727.6 s |
| C-H Deformation | 1452 s | 1614.1 s, 1459.2 s |
| C-C Str. | 1365 s | 1300.8 sh |
| C-N Str. | 1243 s, 1204 m, 1162 s, 1111 m | 1205.9 w, 1104.6 w |
| C-H Bending | 1044 s | 1069.3 w |
| CH2 Rocking | 876 s | 914.6 s, 851.6 w, 757.6 s |
| CNC Deformation | 577 w | 593.3 w |
| υ(Mo=O) of cis-MoO22+ core15 | —- | 983.7 vs, 914.6 s |
H NMR Spectra
1-Methylimidazole,14, 33-35 in solvent CDCl3 has peaks pertaining to CH3 protons at 3.64 ppm. C2-H, C4-H and C5-H have absorptions at 7.38 ppm, 7.01 ppm and 6.86 ppm, respectively. NMR of [1] in solvent DMSO-d6 reveals that peaks due to all the protons of 1-methylimidazole have shifted downfield due to decrease in electronic density of imidazole ring. Effect is inversely proportional to distance (Table-VIII).
Table 8: 1H NMR Chemical Shift in ppm.
| Protons | C3H3N2CH3 (1-Methylimidazole)14, 33-35 in solvent CDCl3 | 1 |
| N-CH3 | 3.64 3H | 3.86 |
| C2-H | 7.38 1H | 9.07 1H |
| C4-H | 7.01 1H | 7.91 1H |
| C5-H | 6.86 1H | 7.63 1H |
1,4-Diaminobutane36, 37 in solvent H2O has peaks pertaining to N-H protons at 1.15 ppm. NMR of [2] in solvent DMSO-d6 indicates that peaks due to NH2 protons and middle CH2 protons of 1,4-diaminobutane have shifted downfield, but peaks due to side CH2 protons have shifted up field (Table-IX) due to N→Mo lone pair donation.
Table 9: 1H NMR Chemical Shift in ppm.
| Protons | H2NC4H8NH2 (1, 4-Diaminobutane)36, 37 in solvent H2O | 2 |
| NH2 | 1.15 4H | 7.87 4H |
| Middle CH2 | 1.74-1.77 4H | 1.99 4H |
| Side CH2 | 3.03-3.06 4H | 2.39-2.41 4H |
2-Methylpyridine21,22,38-40 in solvent CDCl3 has peaks pertaining to CH3, C1-H, C2-H, C3-H & C4-H at 2.54, 7.12, 7.53 & 7.08 ppm, respectively. NMR of [3] in solvent DMSO-d6 reveals that all of these protons have deshielded due to decrease in electron density on N→Mo coordination. There is not much chance of Mo-N π-bonding due to increase of electron density by methyl group on nitrogen (Table-X).
Table 10: 1H NMR Chemical Shift in ppm.
| Protons | C5H4NCH3 (2-Methylpyridine)21, 22, 38-40 in solvent CDCl3 | [3] |
| CH3 | 2.54 3H s | 2.79 3H |
| C2-H | 7.12 1H d | 8.07 1H |
| C3-H | 7.53 1H t | 8.43 1H |
| C4-H | 7.08 1H t | 7.87 1H |
| C5-H | 8.47 1H d | 8.70 1H |
On comparison of NMR of 4-methylpiperidine41 in solvent CDCl3 with that of [4], [5] and [6] (Table-XI), it is found that all peaks in these compounds except that of CH3 have shifted downfield.
Table 11: 1H NMR Chemical Shift in ppm.
| Protons | C5H9NHCH3(4-Methylpiperidine)41 in solvent CDCl3 | [4] | [5] | [6] |
| N-H | 1.84 1H | 8.78-9.02 1H | 8.96-9.20 1H | 8.89-9.13 1H |
| C2-He & C6-He | 3.03 2H | 4.15 2H | 3.46 2H | 3.70 2H |
| C2-Ha & C6-Ha | 2.57 2H | 3.15 2H | 3.14-3.17 2H | 3.16 2H |
| C3-He & C5-He | 1.61 2H | 2.77 2H | 2.74-2.82 2H | 2.78 2H |
| C3-Ha & C5-Ha | 1.08 2H | 1.32 2H | 1.56-1.71 2H | 1.32 2H |
| C4-Ha | 1.45 1H | 2.03 1H | 2.50-2.51 1H | 2.51 1H |
| CH3 | 0.91 3H | 0.88 3H | 0.89 3H | 0.89 3H |
Imidazole35,42,43 in solvent CDCl3 absorbs at 11.62 ppm due to N-H proton. It absorbs at 7.73 ppm due to C-H proton (between two nitrogen atoms) & at 7.15 ppm due to C-H protons on other two carbons. NMR of [7] in solvent DMSO-d6 shows that protons have been deshielded due to decrease in electron density on N→Mo coordination (Table-XII). Due to tautomerization equilibrium, two equivalent C–H protons of imidazole are seen as singlets. N-H proton shows downfield peak.
Table 12: 1H NMR Chemical Shift in ppm.
| Protons | C3H4N2 (Imidazole)35, 42, 43 | [7] |
| N-H | 12.4 1H | 14.93 1H |
| C-H between two nitrogen atoms | 7.70 1H | 9.15 1H |
| C-H on other carbons | 7.03 2H | 7.67 2H |
On comparison of NMR of 1-methylpyrrolidine30,44,45 with that of [8], it is seen that all absorptions show downfield trend due to decrease in electron density on N→Mo coordination (Table-XIII).
Table 13 1H NMR Chemical Shift in ppm.
| Protons | C4H8NCH3 (1-Methylpyrrolidine)30, 44, 45 | [8] |
| CH3 | 2.3 3H | 3.43 3H |
| C2-H & C5-H | 2.5 4H | 2.51-2.89 4H |
| C3-H & C4-H | 1.6 4H | 1.86-1.97 4H |
Mass Spectra (LC-MS)46
Formulae have been derived from fragmentation obtained as under.
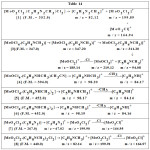 |
Table 14 |
Table 15 Fragments m/z.
| Comp. | Fragment | Theoretical46 | Obtained | Relative area |
| [1] | [MoO2Cl2]+ | 199.83 | 199.89 | 36% |
| [MoO2Cl]+ | 164.86 | 164.94 | 20% | |
| [C3H3N2CH3)]+ | 82.05 | 82.12 | 95% | |
| [3] | [MoOCl4(C5H4NCH3)]+ | 346.83 | 347.20 | 5% |
| [MoOCl3(C5H4NCH3)]+ | 311.86 | 311.20 | 18% | |
| [MoOCl3]+ | 218.80 | 219.12 | 62% | |
| [MoOCl2]+ | 183.83 | 185.14 | 84% | |
| [C5H4NCH3]+ | 93.05 | 94.08 | 100% | |
| [4] | [C5H9NHCH3)]+ | 99.10 | 98.20 | 30% |
| [C5H9NH)]+ | 84.08 | 84.17 | 10% | |
| [5] | [C5H9NHCH3)]+ | 99.10 | 98.17 | 58% |
| [C5H9NH)]+ | 84.08 | 84.14 | 14% | |
| [6] | [C5H9NHCH3)]+ | 99.10 | 98.19 | 15% |
| [C5H9NH)]+ | 84.08 | 84.16 | 6% | |
| [7] | [C3H4N2]+ | 68.03 | 67.02 | 3% |
| [MoO2Cl2]+ | 199.83 | 199.90 | 8% | |
| [MoO2Cl]+ | 164.86 | 164.95 | 5% | |
| [8] | [C4H8NCH3)2]+ | 85.08 | 82.14 | 100% |
| [MoO2Cl2]+ | 199.83 | 199.91 | 9% | |
| [MoO2Cl]+ | 164.86 | 164.97 | 7% |
Acknowledgements
We acknowledge our thanks to Department of SAIF/CIL, Panjab University, Chandigarh (India) for extending us the facility for elemental analysis, FTIR, LC-MS and 1H-NMR to characterize compounds. Our thanks are also to Campus Director, Giani Zail Singh Campus College of Engineering & Technology, Bathinda, Punjab (India), for providing us financial support and infrastructural facilities for this research project.
Conflict of Interest
There is no conflict of interest.
References
- Vasisht, S. K.; Singh, G., VII International Symposium on Organosilicon Chemistry., Kyoto, Japan, 1984, 40.
- Vasisht, S. K.; Singh, G.; Chaudhary, S., Indian Journal of Chemistry., 1985, 24A, 574-577.
- Vasisht, S. K.; Singh, G., Z. Anorg. Allg. Chemie., 1985, 526, 161-167.
- Vasisht, S. K.; Singh, G.; Verma, P. K. Monatshefte fur Chemie., 1986, 117, 177-183.
- Singh, G.; Mangla, V.; Goyal, M.; Singla, K.; Rani, D., American International Journal of Research in Science, Technology, Engineering & Mathematics., 2014, 8(2), 131-136.
- Singh, G.; Mangla, V.; Goyal, M.; Singla, K.; Rani, D., American International Journal of Research in Science, Technology, Engineering & Mathematics., 2015, 9(1), 25-33.
- Singh, G.; Mangla, V.; Goyal, M.; Singla, K.; Rani, D., International Congress on Chemical, Biological and Environmental Sciences., 2015, 930-942, May 7-9, Kyoto (Japan).
- Singh, G.; Mangla, V.; Goyal, M.; Singla, K.; Rani, D., American International Journal of Research in Science, Technology, Engineering & Mathematics., 2015, 10(4), 299-308.
- Singh, G.; Mangla, V.; Goyal, M.; Singla, K.; Rani, D.; Kumar, R., American International Journal of Research in Science, Technology, Engineering & Mathematics., 2016, 16(1), 56-64.
- Singh, G.; Kumar, R., American International Journal of Research in Science, Technology, Engineering & Mathematics., 2018, 22(1), 01-08.
- Mangla, V.; Singh, G., American International Journal of Research in Science, Technology, Engineering & Mathematics., 2019, 26(1), 145-148.
- Vogel, A. I., A Text Book of Quantitative Inorganic Analysis; John Wiley and Sons, New York., 1963. (Standard method).
- https://webbook.nist.gov/cgi/cbook.cgi?ID=C616477&Units=SI&Mask=80.
- Van K. C. G.; Reedijk, J., Inorganica Chimica Acta.,1978, 30, 171-177.
- Abramenko, V. L.; Sergienko, V. S.; Churakov, A. V., Russian J. Coord. Chem., 2000, 26(12), 866-871.
- Ward, B. G.; Stafford, F. E., Inorg. Chem., 1968, 7, 2569.
- Bodo, H. H.; Regina, Z. Chem., 1976, 16, 407.
- Ergu¨ N. Kasap; Su¨ Leyman; O¨ Zceli´K., J. Inclusion Phenomena and Molecular Recognition in Chem., 1997, 28, 259–267.
- http://www.hanhonggroup.com/ir/ir_en/B61062.html.
- Arici K; Gul, O., International J. Chemistry and Technology., 2018, 2(2), 141-152,
- Hossain, A. G. M. M.; Ogura, K., Indian J. Chem., 1996, 35A, 373-378.
- Gupta, S. K.; Srivastava, T. S., J. Inorganic and Nuclear Chem., 1970, 32, 1611-1615.
- https://www.sigmaaldrich.com/spectra/ftir/FTIR008407.PDF.
- Gulluoglu, M. T.; Erdogdu, Y.; Yurdakul, S., J. Molecular Structure., 2007, 834-836. 540-547.
- Fabretti, A. C.; Franchini G. C.; Preti, C.; Tosi, G.; Zannini, P., Transition Metal Chem., 1985, 10, 284-287.
- Manhas, B.S.; Pal, S.; Trikha A. K., Indian J. Chem., 1991, 30A, 638-640.
- Naji A. A.; AL-Askari, M.; Saed, B. A., Basrah Journal of Science (C), 2012, 30, 119-131.
- Mohan, J., Organic Spectroscopy: Principles and Applications, CRC Press., 2004.
- Hodgson, J.B.; Percy, G. C.; Thornton, D.A., J. Molecular Structure., 1980, 66, 81-92.
- Hoa, N. V.; Tuan, N. A.; Thao, P. T.; Huyen, T. T. T., Journal of Science and Technology., 2016, 54(2), 231-237.
- https://www.sigmaaldrich.com/spectra/ftir/FTIR008415.PDF.
- Szafran, M.; Koput, J.; Szafran, Z. D.; Kwiatkowski, J. S., Vibrational Spectroscopy., 2000, 23, 1-11.
- https://www.chemicalbook.com/SpectrumEN_616-47-7_1HNMR.htm.
- http://www.hanhonggroup.com/nmr/nmr_en/B42171.html.
- Zamani, K.; Khaledi, M.; Foroughifar, N.; Mahdavi, V., Turk. J. Chem., 2003, 27, 71-75
- http://www.ymdb.ca/compounds/YMDB00132.
- http://www.hmdb.ca/spectra/spectra/nmr_one_d/1703.
- http://www.hanhonggroup.com/nmr/nmr_en/B61062.html.
- http://www.sigmaaldrich.com/spectra/fnmr/FNMR000256.PDF.
- Kumari, N.; Sharma, M.; Das,, P.; Dutta, D. K., Applied Organomet. Chem., 2002, 16, 258-264.
- https://sdbs.db.aist.go.jp/sdbs/cgi-bin/direct_frame_disp.cgi?sdbsno=591&spectrum_type=HNMR&fname=HSP40452.
- Nuran Özçiçek Pekmez; Muzaffer Can; Attila Yildiza, Acta Chim. Slov., 2007, 54, 131–139.
- http://www.hmdb.ca/spectra/nmr_one_d/1723.
- https://www.sigmaaldrich.com/spectra/fnmr/FNMR010994.PDF.
- http://www1.chem.umn.edu/groups/taton/chem8361/Problem%20Sets/Workshop%201%20Solutions–2012.pdf.
- http://www.sisweb.com/referenc/tools/exactmass.htm.

This work is licensed under a Creative Commons Attribution 4.0 International License.










