Human Hair Keratin Microspheres Prepared by the Water-In-Oil Emulsion Solvent Diffusion Method for Hydrophilic Drug Carrier
Yaowalak Srisuwan and Prasong Srihanam*
Creative Chemistry and Innovation Research Unit, the Center of Excellence in Chemistry, Department of Chemistry, Faculty of Science, Mahasarakham University, Kantarawichai, Maha Sarakham 44150, Thailand.
Corresponding Author E-mail: prasong.s@msu.ac.th
DOI : http://dx.doi.org/10.13005/ojc/350326
Article Received on : 12-03-2019
Article Accepted on : 10-05-2019
Article Published : 18 May 2019
Human hair keratin (HK) was prepared with reducing agent and used in solution to construct microspheres by the simple emulsion solvent diffusion method. The obtained microspheres were observing under scanning electron microscope (SEM). The shape, size distribution and content of microspheres were influenced by W:O ratios. A and 1% w/v keratin solution and 100 mL of oil phase were optimal conditions for fabrication of HK microspheres. The authors studied drug loading efficiency of the HK microspheres by using blue-dextran a model drug and found that the drug loading efficiency as well as releasing profile of blue-dextran were gradually increased by increasing keratin concentration. In conclusion, HK microspheres could be used as hydrophilic carrier molecules for drug delivery system application.
KEYWORDS:Blue-Dextran; Drug Delivery; Emulsion; Human Keratin; Microspheres
Download this article as:| Copy the following to cite this article: Srisuwan Y, Srihanam P. Human Hair Keratin Microspheres Prepared by the Water-In-Oil Emulsion Solvent Diffusion Method for Hydrophilic Drug Carrier. Orient J Chem 2019;35(3). |
| Copy the following to cite this URL: Srisuwan Y, Srihanam P. Human Hair Keratin Microspheres Prepared by the Water-In-Oil Emulsion Solvent Diffusion Method for Hydrophilic Drug Carrier. Orient J Chem 2019;35(3). Available from: https://bit.ly/2JNUIpZ |
Introduction
In recent years, keratin-based materials have been used in many applications1 because of their environmental and safety relative to synthetic materials. The main sources of keratin are epithelial cells,2 human hair, quills, wool, horns, hooves and nails of animals as well as feathers, claws and beaks of birds and reptiles.1 Moreover, previous reports have demonstrated that the keratin protein possesses unique characteristics of bioactivity when properly applied in biomedical and pharmaceutical applications,3,4 water purification, textile finishing and composite materials.5 Drug-loaded materials been attached onto structural proteins such as silk fibroin6,7 and keratin8,9 according their high drug loading efficiency and stability. Microspheres are very interesting for drug delivery applications as their shape is suitable to allow movement in blood vessels. The drug carrier is known as a key factor supporting distribution, solubility and stability especially for water-soluble drugs.7 Microspheres can be constructed by different techniques included emulsion solvent extraction/evaporation, emulsion core template extraction, spray drying and electro spraying,10 emulsion, phase separation, precipitation and gelation.11-16 There are some limitations with the delivery of water-soluble drugs or biological molecules such as enzymes or proteins due to their sensitivity to metabolic processes in the body as well as physical-chemical treatments both during processing or post treatment. Many reports have proposed encapsulation of biological molecules.17-19
Herein, the authors present an emulsion solvent diffusion method for human keratin microspheres preparation with and without blue-dextran, a sample hydrophilic drug entrapment. The suitable conditions concerning microspheres processing were investigated and were discussed. In addition, drug release profile from the HK microspheres was determined in vitro.
Materials and Method
Human hair was collected, washed and dried in room temperature. Hair was immersed in n-hexane for 12 h to remove some lipids. Keratin from human hair was extracted following the method of Shindai.20 The hair sample was mixed with a solution of 8 M urea, 0.26 M SDS, 0.4 M NaOH in 100 mL distilled water at 70°C and stirred until the hair was dissolved. The solution was then dialyzed for 3 days in distilled water using cellulose tubular membranes (molecular weight cut-off 3.5 kDa, Thermo Scientific, USA). Finally, the human keratin (HK) solution was checked by evaporation technique and the concentration adjusted with distilled water to obtain a 2% (w/v) solution.
Construction of Human Keratin Microspheres
HK microspheres were constructed according to the method of Imsombut et al.6 with varied solutions of HK and volumes of ethyl acetate and of W:O phase ratios.
Preparation of Blue Dextran-loaded HK Microspheres
The best condition for preparing of HK microsphere was chosen to prepare blue dextran-loaded HK microspheres. Briefly, the model water-soluble drug, blue dextran was dissolved in the HK solution (HK:drug at 4:1 (w/w)) before construction the microspheres. The different volume of HK solution mixed blue dextran (1.5, 1.0 and 0.5 mL) was drop-wise into 100 mL ethyl acetate with stirring at 800 rpm for 20 min. The microspheres were collected by centrifugation before drying in vacuum oven at room temperature.
Morphological observation
Scanning electron microscopy (SEM, JEOL JSM-6460LV) was used to investigate the morphology and size distribution of the HK microspheres. Before observation, the SF microspheres were coated with gold for enhancing conductivity.
Releasing Profile of Blue-Dextran In Vitro Test
The blue dextran-loaded HK microspheres were immersed in phosphate buffer saline (PBS) pH 7.4 at 37°C to test releasing profile over time. The content of blue dextran contained in buffer was measured by absorbance at 620 nm using a standard calibration curve. Pure HK microspheres were used as a control. Drug loading efficiency percentage (%DLE) (3) was calculated from theoretical drug loading content (DLCtheoretical) (1) and actual drug loading content (DLCactual) (2) as follows;
DLCtheoretical (%) = {feed drug (mg)/feed HK + feed drug (mg)}x 100 (1)
DLCactual (%) = {entrapped drug (mg)/drug-loaded microspheres (mg)} x 100 (2)
DLE (%) = (DLCactual/DLCtheoretical) 100 (3)
Results and Discussion
Morphological Determination of HK Microspheres
The emulsion solvent diffusion method has attracted recent interest in its use for making microspheres due to it being a single step process without further treatment such as heat or alcohol treatment. Moreover, it can be loaded with target materials into protein before the microsphere formation process. The solubility of ethyl acetate in water is approximately 8.3-7.15 g/100 mL (or 0.0715-0.083% (w/v) depending on processing temperature.21 The results indicated that HK microspheres can be made by the emulsification-diffusion process which as noted above, using little process equipment and with low consumption of chemical materials. The ethyl acetate (oil phase) diffused into hydrophilic phase (water) and resulted in the formation of microspheres by decreasing of keratin polarity.7,10
The yields of HK microspheres varied by the ratio of HK solution used and the particle sizes of the HK microspheres was slightly increased by decreasing W:O ratios. Fig.1 shows HK microspheres prepared by using 0.5 mL of 0.5%w/v keratin solution in 100 mL of ethyl acetate. Under these conditions, the obtained HK microspheres have several shapes with some pores in their surfaces. The consistency in shape and size of microspheres was increased with an oil (ethyl acetate) volume of 200 mL was used, as shown in Fig. 2. The microspheres have hollow pores and rough surfaces at high magnification. These conditions gave a low content of HK microspheres. The contents of HK microspheres increased when keratin concentration was increased (1.0%w/v) as shown in Fig. 3. However, the obtained HK microspheres aggregated together as well occurring as separate particles. The separate microspheres had smooth surfaces and also were dense in their texture. To find the suitable W:O ratios, increasing the volume of keratin solution from 0.5 to 1.0 mL was performed. The results showed that the HK microspheres were large in size and separate microspheres are shown in Fig. 4. With increasing oil volume (200 mL), the obtained HK microspheres have more consistency in shapes and large size than under other conditions as shown in Fig. 5. However, some microspheres with small grooves on their surfaces also appeared. It was surprising that using a high volume of keratin solution (2 mL) resulted in a low content of microspheres with incomplete shapes that were varied in size. Imbalance between the water and oil (W:O) phase resulted in aggregation of microspheres as shown in Fig. 6. This may have been caused by functional group of amino acid in keratin protein reacting together with water and retarding diffusion of water into oil phase.14 In case of incomplete microspheres, they may have been caused by using low content of keratin which was insufficient to create enclosed microspheres. On the other hand, flow of keratin solution according from disulfide bonds in keratin protein should be caused of not enclosed microspheres. Furthermore, the hollow or porous microparticles may have been caused by diffusion of high-water content out from inside to external emulsion droplets, while roughness in surface might have arisen from separation of water and oil phases during the diffusion process before microsphere formation.22,23
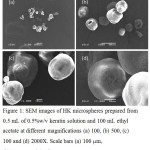 |
Figure 1: SEM images of HK microspheres prepared from 0.5 mL of 0.5%w/v keratin solution and 100 mL ethyl acetate at different magnifications (a) 100, (b) 500, (c) 100 and (d) 2000X. Scale bars (a) 100 mm, (b) 50 mm, and (c, d) 10 mm. |
 |
Figure 2: SEM images of HK microspheres prepared from 0.5 mL of 0.5%w/v keratin solution and 200 mL ethyl acetate at different magnifications (a) 100, (b) 500, (c) 100 and (d) 2000X. Scale bars (a) 100 (b) 50 and (c, d) 10 mm. |
 |
Figure 3: SEM images of HK microspheres prepared from 0.5 mL of 1.0 %w/v keratin solution and 100 mL ethyl acetate at different magnifications (a) 100, (b) 500, (c) 100 and (d) 2000X. Scale bars (a) 100, (b) 50, and (c, d) 10 mm. |
 |
Figure 4: SEM images of HK microspheres prepared from 1.0 mL of 1.0%w/v keratin solution and 100 mL ethyl acetate at different magnifications (a) 100, (b) 500, (c) 100 and (d) 2000X. Scale bars (a) 100, (b) 50, and (c, d) 10 mm. |
 |
Figure 5: SEM images of HK microspheres prepared from 1.0 mL of 1.0%w/v keratin solution and 200 mL ethyl acetate at different magnifications (a) 100, (b) 500, (c) 100 and (d) 2000X. Scale bars (a) 100, (b) 50, and (c, d) 10 mm. |
 |
Figure 6: SEM images of HK microspheres prepared from 2.0 mL of 1.0%w/v keratin solution and 200 mL ethyl acetate at different magnifications (a) 100, (b) 500, (c) 100 and (d) 2000X. Scale bars (a) 100, (b) 50, and (c, d) 10 mm. |
Drug Loading Efficiency and Releasing Profiles
Table 1 shows drug loading efficiency of blue-dextran-loaded HK microspheres in vitro. The results showed that the DLE values increased by increasing the content of keratin. This indicated that keratin could be used as drug carrier for a hydrophilic drug and also adjusted the loading efficiency by varying the content of keratin. Although keratin is a class of structural protein, it has a high content of hydrophilic amino acids such as cysteine, aspartic acid, glutamic acid, arginine, serine and threonine.9 The release patterns of blue-dextran from HK microspheres were as shown in Fig. 7. After 6 h of releasing in PBS, the microspheres prepared from high content of keratin (1.5% w/v) showed the lowest drug releasing content (Fig. 7c), while drug releasing content was found to be the highest in 1% w/v keratin solution microspheres. At the initial time of the releasing experiment, the highest drug releasing content was found in the microspheres prepared from 0.5%w/v keratin solution and gradually increased until 6 h of releasing time. The 1.5% w/v keratin microspheres showed similar drug releasing pattern with 1.0%w/v keratin microspheres while the drug releasing pattern of 1.0%w/v microspheres was dramatically different. This was due to the 0.5%w/v microsphere has rapidly increased of drug release content after 1 h of investigation until the final time. This might be from the burst release effect of blue dextran on the surfaces of microsphere while other conditions might be distributed covering the texture of microsphere.
Table 1: Drug loading efficiency (DLE) of blue-dextran loaded-HK microspheres.
|
Keratin concentration (%w/v) |
% DLCactual |
%DLCtheoretical |
%DLE |
|
0.5 |
3.50 ± 1.05 |
9.09 ± 0.55 |
38.50 ± 2.55 |
|
1.0 |
7.10 ± 1.30 |
13.04 ± 2.10 |
54.44 ± 3.60 |
|
1.5 |
20.50 ± 2.06 |
20.00 ± 2.45 |
102.50 ± 5.73 |
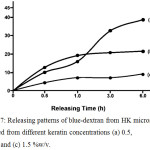 |
Figure 7: Releasing patterns of blue-dextran from HK microspheres prepared from different keratin concentrations (a) 0.5, (b) 1.0 and (c) 1.5 %(w/v) |
Conclusion
This work proved that the W/O emulsion solvent diffusion method was practically useful for making HK microspheres and also hydrophilic drug-loaded HK microspheres. The sizes and shapes of the microspheres varied according to the volume of keratin solution used and the W:O phase ratio. The drug loading efficiency as well as drug releasing profiles depended on the concentration of keratin used for microsphere preparation. The obtained HK microspheres are expected to be potentially useful a carrier for water-soluble drug delivery applications.
Acknowledgements
This Research was Financially Supported by Mahasarakham University Grant Year 2018. We also expressed to Faculty of Science, Mahasarakham University for SEM observation. In addition, we would like to thank the Center of Excellence for Innovation in Chemistry (PERCH-CIC), Commission on Higher Education, Ministry of Education, Thailand for partial financial support.
References
- Wang, B.; Yang, W.; McKittrick, J.; Meyers, M. A. Prog. Mater. Sci. 2016, 76, 229.
- Coulombe, P. A.; Omary, M. B. Curr. Opin. Cell Biol. 2002, 14, 110.
- Wang, J.; Hao, S.; Luo, T.; Cheng, Z.; Li, W.; Gao, F.; Guo, T.; Gong, Y.; Wang, B. Colloids Surf. B. 2017, 149, 341.
- Luo, T.; Hao, S.; Chen, X.; Wang, J.; Yang, Q.; Wang, Y.; Weng, Y.; Wei, H.; Zhou, J.; Wang, B. Mater. Sci. Eng. C, 2016, 63, 352.
- Ma, B.; Qiao, X.; Hou, X.; Yang, Y. Int. J. Biol. Macromol. 2016, 89, 614.
- Imsombut, T.; Srisuwan, Y.; Srihanam, P.; Baimark. Y. Powder Technol. 2010, 203, 603.
- Srisuwan, Y.; Baimark, Y.; Srihanam, P. Particul. Sci. Technol. 2017, 35, 387.
- Srisuwan, Y.; Srihanam, P. Adv. Mater. Sci. Eng. 2018, 2018, 1.
- Rajabinejada, H.; Patrucco, A.; Caringella, R.; Montarsolo, A.; Zoccola, M.; Pozzo, P. D. Ultrason. Sonochem. 2018, 40, 527.
- Baimark, Y.; Srihanam, P.; Srisuwan, Y.; Phinyocheep, P. J. Appl. Polym. Sci. 2010, 118, 1127.
- O’Donnell, P. B.; McGinity, J. W. Adv. Drug Deliv. Rev. 1997, 28, 25.
- Freytag, T.; Dashevsky, A.; Tillman, L.; Hardee, G. E.; Bodmeier, R. J. Control Release. 2000, 69, 197.
- Wieland-Berghausen, S.; Schote, U.; Frey, M.; Schmidt, F. J. Control Release. 2002, 85, 35.
- Dowding, P. J.; Atkin, R.; Vincent, B.; Bouillot, P. Langmuir. 2004, 20, 1374.
- Yeo, Y.; Park, K. J. Control Release. 2004, 100, 379.
- Manju, S.; Sreenivasan, K. Colloids Surf. B. 2011, 82, 588.
- Sinha, V. R.; Trehan, A. J Control Release. 2003, 90, 261.
- Malafaya, P. B.; Silva, G. A.; Reis, R. L. Adv. Drug. Deliv. Rev. 2007, 59, 207.
- Wang, X.; Wenk, E.; Hu, X.; Castro, G. R.; Meinel, L.; Wang, X.; Li, C.; Merkle, H.; Kaplan, D. L. Biomaterials. 2007, 28, 4161.
- Nakamura, A.; Arimoto, M.; Takeuchi, K.; Fujii, T. Biol. Pharm. Bull. 2002, 25, 569.
- Srisa-ard, M.; Baimark, Y. Particul. Sci. Technol. 2013, 31, 379.
- Altshuller, A. P.; H. E. Everson, H.E. J. Am. Chem. Soc. 1953, 75, 1727.
- Cheerarot, O.; Baimark, Y. e-Polymers. 2015, 15, 67.

This work is licensed under a Creative Commons Attribution 4.0 International License.









