Green Synthesis, Characterisations and Antimicrobial Activities of CaO Nanoparticles
Bharti Ramola, Naveen Chandra Joshi* , Monika Ramola, Juhi Chhabra and Ajay Singh
, Monika Ramola, Juhi Chhabra and Ajay Singh
Department of Chemistry, Uttaranchal University Dehradun, India.
Corresponding Author E-mail: drnaveen06joshi@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350333
Article Received on : 13-03-2019
Article Accepted on : 10-05-2019
Article Published : 21 May 2019
The conventional methods used to synthesise organic and inorganic compounds are suffering from high cost, not eco-friendly, low efficient and not more suitable in large scale operations. Recently, we need an efficient and environmentally-friendly synthetic approach to synthesise some important inorganic or organic compounds in nano scales. Green processes possess the minimization of harmful chemicals and instrumentations, low cost, simple, no harmful chemical generations and high efficiency. In nano-scale, the potential of the compounds significantly increases and the green synthetic approaches in synthetic chemistry are better alternatives and efficient over conventional methods. In the present study, we have synthesised calcium oxide (CaO) nanoparticles by using the leave extract of Rhododendron arboreum. Calcium oxide nanoparticles are found valuable in adsorption, antimicrobial activities, catalysis and adsorption. The freshly synthesised nanoparticles have been characterised by different analytical methods such as UV-Visible, FT-IR, XRD, FESEM and EDX. The CaO nanoparticles are applicable in the antimicrobial activities against Escherichia coli, Streptococcus mutans and Proteus vulgaris and significant zone inhibitions found over these microorganisms in the pattern E. coli > S. mutans > P. vulgaris.
KEYWORDS:Antimicrobial Activities; CaO Nanoparticles; Characterisations; Green Synthesis
Download this article as:| Copy the following to cite this article: Ramola B, Joshi N. C, Ramola M, Chhabra J, Singh A. Green Synthesis, Characterisations and Antimicrobial Activities of CaO Nanoparticles. Orient J Chem 2019;35(3). |
| Copy the following to cite this URL: Ramola B, Joshi N. C, Ramola M, Chhabra J, Singh A. Green Synthesis, Characterisations and Antimicrobial Activities of CaO Nanoparticles. Orient J Chem 2019;35(3). Available from: https://bit.ly/2QdDC65 |
Introduction
Nanotechnology is the branch of science concerned with the performance of atoms or molecules under nano scales (1 to 100 nanometres). The products consisted such constituents are smaller, lighter, durable, stronger and with a large surface area and unique physical and chemical properties.1 One of the major applications of nanotechnology in chemistry is the synthesis of metal and metal oxide nanoparticles.2 The main purposes of adopting a green synthetic approach to synthesise nanoparticles of a variety of metal and metal oxides are minimizations of hazardous chemicals, prevention of wastes, efficiency, low cost and comparatively high yield of products3. The common inorganic nano metal oxides are magnesium oxide (MgO), titanium oxide (TiO2), copper oxide (CuO), zinc oxide (ZnO) and calcium oxide (CaO). These nano metal oxides are safe to human and other organisms, stable, antimicrobial agents and have multifunctional properties4. Calcium oxide (CaO) nanoparticles or CaO NPs have significant antimicrobial properties and unique structural and optical properties; environmentally, they are safe to all living organisms.5-7 Due to specific structural and optical properties, calcium oxide nanoparticles are used as potential drug delivery agents in photo thermal and photodynamic therapy and synaphic delivery.8 With potential biomedical applications, CaO NPs are also used in the various fields such as electronics, environmental remediation, sensors and catalysis.9 The overall synthetic process utilising leave extract of Rhododendron arboreum is very cheap, reliable and no needed any additional charges on instrumentations/chemicals.10 The plant Rhododendron arboreum is an Angiospermic plant belongs to family Ericaceae. It is evergreen tree growing upto 21 m tall and also known as burans. Leaves are oblong-lanceolate and about 9-18 cm long and 10-18 cm wide. Green leaves of the plant contain a-amyrin, epifriedelinol, ericolin, glucoside and urosolic acid.11 The leaves of Rhododendron arboreum were collected from the Kumaun hills of Uttarakhand (India).
Materials and Method
All the chemicals used in the experimental section were of Analytical grade and deionised water used to wash all the glassware and prepare necessary solutions. The collected leaves of Rhododendron arboreum have been washed 2-3 times with deionised water then dried completely at room temperature for 2 days. The dried leaves cut into possible small pieces and 10 g of cut leaves were boiled with 100 ml of deionised water for 45 minutes on a magnetic stirrer. The content was filtered with whatmann (no 1) filter paper and the filtrate used as an extract and the prepared extract preserved at 40C for further uses. Take 50 ml of leaf extract and 50 ml of calcium nitrate Ca(NO3)2 solution in an Erlenmeyer flask (250 ml). The content has been placed on magnetic stirrer for 40 minutes and added 0.1N sodium hydroxide (NaOH) solution drop wise in the reacting mixture. Now, the reaction mixture was centrifuged at 10,000 rpm for 15 minutes and the precipitate settled down. This precipitate was dried at hot air oven for one hour and then washed carefully to remove the basicity. Further calcination has been carried out in muffle furnace for three hours at 5000C temperature. The freshly prepared nanoparticles were preserved in an air tight sample tube for further studies and characterisations. The characterisation methods included FT-IR, UV-Vis, Powder XRD, FESEM and EDX.
Results and Discussion
Characterisations of CaO NPs
FT-IR and UV-Visible Spectroscopy
Infra-red spectroscopy is one of the powerful techniques to provide a basic idea on molecular vibrations or transitions in vibrational and rotational levels in molecules. Fourier transform infrared spectroscopy (FT-IR) basically collects the high resolution data over broad spectral ranges. This spectroscopy is depending on the interference of electromagnetic radiation and provides the information of the different functional groups present on the surface of nanomaterials.12 The FT-IR spectra have been obtained in the range 4000 to500 cm-1 (Fig.1). Broad peaks are found at 3242 cm-1, 2514 cm-1, 2138 cm-1, 1797 cm-1, 1635 cm-1, 1430 cm-1, 1156 cm-1, 1074 cm-1, 876 cm-1 and 600 cm-1. These peaks indicate the presence of –N-H, -O-H (Carboxylic), -CN, C=O, C=N, C=C, C-O, C-C, Ca-O (linkages) etc in the nano-powder of calcium oxide. UV-visible spectroscopy is very common and useful tool in analytical chemistry. This tool is basically used for the identification of chemical products and their qualitative analysis. The molecules or atoms absorb UV-Visible radiation and their electrons excited from lower energy levels to higher energy levels. In the present study, we have obtained the UV-Visible spectra in the range of wavelength 260-410 nm (Fig.2). The broad peaks have obtained at 265 and 350 nm; this indicating the formation of calcium oxide nanoparticles at such wavelength.
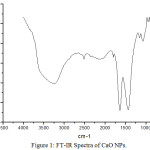 |
Figure 1: FT-IR Spectra of CaO NPs. |
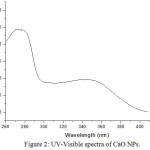 |
Figure 2: UV-Visible spectra of CaO NPs. |
Powder-XRD, FESEM and EDX
X-ray diffraction (XRD) is very essential non-destructive analytical tool to characterise all matters like fluids, powder and crystals. XRD technique is based on the diffraction of X-rays on powder or crystalline materials and in the diffractogram, the diffracted intensity is represented with scattered two theta (2q) angles. The XRD-pattern of CaO NPs is represented in figure 3 and all the observed peaks are found in good agreement with standard JCPDS data (00-004-0777). It indicates the cubic phase and some calcite peaks are due to the carbonation reaction of calcium oxide with free carbon dioxide.8
Field Emission Scanning Electron Microscope (FESEM) works with negatively charged electron and the sample is scanned by these electrons with zig-zag patterns. Electrons are released from a field emission source and at high vacuum column these are focussed and deflected by electronic lenses. The speed and angles of electrons vary with surface structure of materials. In general, FESEM is utilised in the identification of morphology of nanomaterials and the FESEM images of CaO NPs are represented in figure 4. These images indicate a broad needle like morphology of calcium oxide nanoparticles synthesised by using the leaf extract of Rhododendron arboreum. Energy Dispersive Spectroscopy (EDS) generally involves the generation of X-ray spectrum from the scanning area of FESEM. It represents the elemental composition in the materials (Fig. 5 and Table 1).
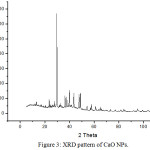 |
Figure 3: XRD pattern of CaO NPs. |
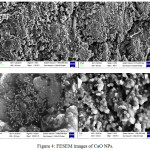 |
Figure 4: FESEM images of CaO NPs. |
Table 1: EDX Analysis of CaO NPs.
|
Element |
Weight % |
Atomic % |
|
C K |
6.56 |
10.55 |
|
O K |
61.28 |
73.96 |
|
Ca K |
32.16 |
15.49 |
|
Total |
100.00 |
|
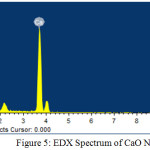 |
Figure 5: EDX Spectrum of CaO NPs. |
Antimicrobial Activities
The nanoparticles of CaO show good antibacterial properties because of large surface area and the reduced particle size increases the reactivity of CaO NPs with pathogenic bacteria. Due to smaller size, these nanoparticles can easily enter in the bacterial cells and an inhibition mechanism proceeds inside the bacterial cell. After entering in the bacterial cell, a CaO nanoparticle leads the distortion and destroys the cell membrane that causes the death of cells.13 Agar well diffusion method was applied to perform the antimicrobial activity of CaO NPs against the bacterial strains i.e. Escherichia coli, Streptococcus mutans and Proteus vulgaris. At very low concentration of CaO nanoparticles (1mg/ml), a significant zone inhibitions for such bacteria have been obtained; 16.6 mm for E. coli, 14 mm for S. mutans and 12 mm for P. vulgaris (Table 2).
Table 2: Antimicrobial activity of CaO NPs.
|
Bacteria |
Zone inhibitions (mm) at 1mg/ml |
|
E. coli |
16.6 |
|
S. mutans |
14 |
|
P. vulgaris |
12 |
Conclusion
In the present study, we have obtained CaO NPs with a good yield using the leaf extract of Rhododendron arboreum in very low cost. The synthesised nanoparticles were well analysed by using different analytical methods i.e. FTIR, UV-Vis, XRD, FESEM and EDX. All analytical methods indicate the formation of CaO nanoparticles under a laboratory scale. These nanoparticles are very effective at low concentrations for the antimicrobial activities against the bacterial strains i.e. Escherichia coli, Streptococcus mutans and Proteus vulgaris.
Ackowledgements
I am thankful to the Department of Chemistry, Uttaranchal University Dehradun (India) for providing all facilities during the experimental works.
Conflict of Interest
There is no conflict of interest.
References
- Joshi, N.C.; Singh,A. I.J.G.H.C., 2018, 7, 467-475.
- Rotello, V.M., Nanoparticles: Building Blocks for Nanotechnology; Springer Science & Business Media: New York,NY, USA, 2004.
- Saif, S.; Tahir, A.; Chen, Y. Nanomaterials, 2016, 6, 209-236.
- Bae, D.H.; Yeon, J.H.; Park, S.Y. Archives of Pharmacal Research, 2006, 29, 298-301.
- Wang, L.; Hu. C.; Shao, L. International J. of Nanomedicine, 2017, 12, 1227-1249.
- Sawai, J. J. Microbiological Methods, 2003, 54, 177-82.
- Butt, A.R.; Ejaz, S.; Baron, J.C.; Ikram, M.; Ali, S. Digest J. of Nanom. and Biost., 2015, 10, 799 – 809.
- Abraham, S.; Sarathy, V.P. Int. J. Pharm. Sci. Rev. Res., 2018, 49, 121-125.
- Balaganesh, A.S.; Sengodan, R.; Ranjithkumar, R.; Chandarshekar, B. International J. of Innov. Techn.Expl. Engin., 2018, 8, 312-314.
- Jagadeesh, D. Prashantha, K.; Shabadi, R. 2017, 47, 708-712.
- Srivastava, P. J. Applied Pharm. Sci. 2012, 2, 158-162.
- Joshi, N. C.; Singh, A. Rajput, H.; Orient. J. Chem., 2018, 34, 2548.
- Mohammed, D.; Aziz, A.E.; Naeima; Yousef, M. H. ARC J. Animal and Veterinary Scien., 2017, 3,38-45.

This work is licensed under a Creative Commons Attribution 4.0 International License.









