Development of Chitosan-TiO2 Nanocomposite for Packaging Film and its Ability to Inactive Staphylococcus Aureus
Indar Kustiningsih* , Asep Ridwan, Devi Abriyani, Muhammad Syairazy, Teguh Kurniawan and Dhena Ria Barleany
, Asep Ridwan, Devi Abriyani, Muhammad Syairazy, Teguh Kurniawan and Dhena Ria Barleany
Department of Chemical Engineering, Faculty of Engineering, University of Sultan Ageng Tirtayasa, Serang, 42118, Indonesia.
Corresponding Author E-mail: indar.kustiningsih@untirta.ac.id
DOI : http://dx.doi.org/10.13005/ojc/350329
Article Received on : 14-03-2019
Article Accepted on : 14-05-2019
Article Published : 18 Jun 2019
The aim of present study is to synthesize chitosan-TiO2 nanocomposite for packaging and its ability to inactive Staphylococcus aureus. TiO2 Degussa P25 were dispersed in chitosan matrix in order to produce film-forming solution. The samples were characterized by SEM, FTIR, tensile strength, antibacterial and biodegradable test. The tensile strength test results showed that Cs-0.1Ti was the best nanocomposite compared to other variations of TiO2 addition. Whereas for the test of S. aureus bacteria showed that no more S. aureus bacteria were found in chitosan-TiO2 nanocomposite after incubation for 24 hours. It indicated more effective use of nanocomposites by adding TiO2 compared to without adding TiO2. For biodegradation analysis, the addition of TiO2 slows the nanocompostites degradation process, which is indicated by the less mass loss that occurs in Cs-0.5 Ti nanocomposite.
KEYWORDS:Biocomposite; Biodegradation; Chitosan; Food Packaging; Staphylococcus Aureus; TiO2
Download this article as:| Copy the following to cite this article: Kustiningsih I, Ridwan A, Abriyani D, Syairazy M, Kurniawan T, Barleany D. R. Development of Chitosan-TiO2 Nanocomposite for Packaging Film and its Ability to Inactive Staphylococcus Aureus. Orient J Chem 2019;35(3). |
| Copy the following to cite this URL: Kustiningsih I, Ridwan A, Abriyani D, Syairazy M, Kurniawan T, Barleany D. R. Development of Chitosan-TiO2 Nanocomposite for Packaging Film and its Ability to Inactive Staphylococcus Aureus. Orient J Chem 2019;35(3). Available from: https://bit.ly/2MUXwF1 |
Introduction
Plastic is widely used as food pacaking due to elastic, lightweight, not easily broken, transparent, waterproof and easy to carry. However, plastics made from petroleum has led to serious ecological problems due to their non biodegradability.1,2 In the recent years, there has been an increasing interest in developing biodegradable plastics from renewable resources.
One of renewable plastic is bioplastic because the compounds inside are derived from plants like cellulose, lignin, starch and animal like casein, protein, lipid and chitosan.3 Chitosan represents biodegradable4,5 and biocompatible cationic polysaccharide with premium film forming ability, mechanical strength, flexibility and also a non toxic.4,6 Unfortunately, bioplastic from chitosan has a disadvantage that is its lower mechanical properties compared to fossil based plastics.7 A modification in the form of nanocomposite can be a good solution to improve the mechanical properties of chitosan-based bioplastic.8
The synthesis of nanocomposite was carried out by incorporating nanofillers such as silica, clay, and titanium dioxide (TiO2) in chitosan which made it possible not only to improve mechanical and barrier properties but also to provide other functions in food packaging applications.9,10 TiO2 is a photocatalyst and has been widely utilized in many applications such as hydrogen generation, water purification, and decomposition of pollutants, air purification, and also in medical applications, self cleaning and self-disinfecting materials.11-15 TiO2 nanoparticle has been used as an effective antibacterial agent because its widespread availability, broad-spectrum antibiosis and low cost. It has excellent physic-chemical properties, physical and chemical stability, good dispersing properties, strong oxidizing power, fast electron transfer rate and good biocompatibility.16,17
The migration of the active compounds into the food stuff is the major disadvantage of antimicrobial package. This is especially undesirable, when the general trend is to limit the presence of additives in processed food. Therefore, chitosan and TiO2 are ideal candidates for food packaging due to its nontoxicity. Furthermore, there are few reports on the synergistic effect between TiO2 nanoparticles and chitosan regarding its biodegradability and antibacterial activity especially on Staphylococcus aureus. The aim of this study was to prepare chitosan-TiO2 nanocomposite and to characterize these films for their structure, morphology, mechanical properties and antibacterial activity.
Materials and Method
Synthesis of Chitosan-TiO2 Nanocomposite
1 g of chitosan (DD 85-89% Biochitosan) was added to 1% (v/v) 100 mL glacial acetic acid. The mixture was stirring using an overhead stirrer for 3 hours at room temperature. Then, the solution was homogenized for 30 minutes. Furthermore, TiO2 nanoparticles were added with variations in the composition (0 g, 0.1 g, 0.2 g, 0.5 g, and 1 g). The solution was stirred using an overhead stirrer for 4 hours at room temperature and then the solution was homogenized for 1 hour. The solution was poured into a glass plate and dried at a temperature of 80°C. After obtaining bioplastic, SEM characterization, FTIR analysis, mechanical strength test, antibacterial activity test and biodegradable plastic analysis were carried out.
Biodegradability Analysis
Biodegradation analysis was carried out by the method of sample burial in a mixture of soil and compost. The simplest quantitative method to determine the biodegradation of a polymer is by measuring its mass loss. Mass loss measurement was done by weighing the polymer mass before and after the biodegradation process for a certain time interval. In this study, six plastic samples were used with dimensions of 4 x 4 cm and weighed regularly 1 week for 2 months.
Antimicrobial Activity
Staphylococcus aureus was first rejuvenated, then the NA media and nanocomposite with predetermined variations were sterilized. Serial dilution of Staphylococcus aureus was performed by using of 103 and 104 dissolution. After that, the media and bacteria were poured on the petri dish. The process was followed by incubation for 24 hours in a dark room with UV irradiation. After the incubation, the number of bacterial colonies were calculated by CFU method (colony forming units).
Results and Discussion
Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
FTIR is a useful technique for the detection of chemical bonds in the materials. The FTIR spectra of chitosan and chitosan-TiO2 nanocomposites are shown in Figure 1. It shows the similarity of the resulting spectrum.
 |
Figure 1: FTIR pattern of chitosan bioplastic and chitosan-TiO2 nanocomposites. |
Figure 1 shows the similarity of the resulting spectrum. This similarity shows that most functional groups owned by Cs are also owned by Cs-TiO2 bioplastics. If two ingredients are mixed (Cs and TiO2), a physical mixture can be formed and even chemical interactions can occur which are characterized by characteristic changes at the peak of the spectrum of bioplastic analysis results on FTIR. Compared to pure chitosan (Cs), hydroxyl bonds, amino and amide groups experience changes in the Cs-Ti spectrum. It indicated there are interaction between Cs and TiO2.4 The interaction of Cs and TiO2 is characterized by the formation of Cs-TiO2 bonds in the wavelength range of 450-950 cm-1.
Figure 1 shows that Cs spectrum had OH and NH functional groups which absorption peaks at wavelengths 3419.79 cm-1, CH groups at wavelengths 2924.09 cm-1, groups C=O at wavelengths 1616.35 cm-1, CO group at wavelength 1257.59 cm-1, and CN group at wavelength 1039.63 cm-1. While the Cs-Ti spectrum was a combination of the peaks of chitosan and TiO2 groups. It indicated that there was a cross-linking process between chitosan and TiO2. The OH functional group overlaps with NH at a wavelength of 3439.08 cm-1, the R-NH-R group at a wavelength of 1600.92 cm-1, the deformation group OH at a wavelength of 1442.75 cm-1, the deformation group Cs-TiO2 at a wavelength of 947.05 cm-1.
Scanning Electron Microscopy (SEM) Characterization
In an attempt to study microstructural changes in the Cs-Ti nanocomposites, SEM was conducted to visualize the surface topography for different samples. The SEM images of chitosan bioplastic (Cs), nanocomposite chitosan-0.1 g TiO2 (Cs-0.1Ti), chitosan-0.2 g TiO2 (Cs-0.2 Ti), chitosan-0.3 g TiO2 (Cs-0.3 Ti), chitosan-0.5 g TiO2 (Cs-0.4 Ti) and chitosan-0.5 g TiO2 (Cs-0.5 Ti) can be seen at Figure 2. Addition of TiO2 on bioplastic strongly influenced the morphology of the Cs-Ti nanocomposites as evidenced in Figure 2.
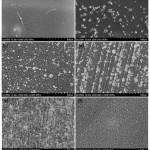 |
Figure 2: SEM images of (a) Cs (b) Cs-0.1 Ti (c) Cs-0.2 Ti (d) Cs-0.3Ti (e) Cs-0.4 Ti (f) Cs-0.5 Ti. |
Figure 2(a) shows bioplastics without the addition of TiO2 have homogeneous, smooth and clean morphology without granules. It is different to the SEM images of Cs-Ti nanocomposites. Figure 2 (b-e) indicated that the addition of TiO2 created morphology of bioplastic had an irregular granule distribution which shows the distribution of TiO2 in the matrix. SEM images of nanocomposites Cs-Ti shows irregular shapes of TiO2 distribution in the matrix.
In Figures 2(b) to Figure 2(f) show that the surface of the nanocomposite had a homogeneous, less smooth surface, granules and looks less than perfect. The irregularity of the nanocomposite surface was due to the addition of TiO2 in the chitosan matrix. It indicated that the distribution of granules in the matrix increases with the addition of TiO2. It can be seen in Figure 2(f) that Cs-0.5Ti had denser granule distribution than the other nanocomposites with different TiO2 compositions. A study reported that the homogeneous dispersion in matrix polymers is one of the most important factors in creates a good composite performance.18
Tensile Strength and Elongation
The effect of TiO2 addition on nanocomposites to the value of tensile strength (TS) and elongation (E) can be seen at Table 1.
Table 1: The effect of TiO2 addition on nanocomposites to the value of tensile strength and elongation.
| No | Mixture | Tensile Strength (MPa) | Error of TS | Elongation (%) | Error of elongation |
| 1 | Cs | 63.86 | ±0.001 | 4.66 | ±0.0001 |
| 2 | Cs-0.1Ti | 12.35 | ±0.001 | 2 | ±0.0001 |
| 3 | Cs-0.2Ti | 10.21 | ±0.001 | 1.66 | ±0.0001 |
| 4 | Cs-0.3Ti | 7.85 | ±0.001 | 0.59 | ±0.0001 |
| 5 | Cs-0.4Ti | 4.97 | ±0.001 | 0.57 | ±0.0001 |
| 6 | Cs-0.5Ti | 0.24 | ±0.001 | 0.12 | ±0.0001 |
Table 1 shows the addition of TiO2 on nanocomposites lead to the decreasing value of TS and E. The results showed the highest TS and E at the addition of 0.1 gr TiO2 (Cs-0.1 Ti). This decrease was due to the presence of granules in the matrix which caused the matrix to be less homogeneous, whereas to get a large TS, a homogeneous molecular structure was needed. The higher the addition of TiO2 caused the composition of chitosan which is originally homogeneous to change its molecular structure to become irregular.
Biodegradable Analysis
Samples (4x4cm) were tested for biodegradation by measuring their weight every week. A decrease in mass from nanocomposites can be seen in Table 2 and Figure 3.
Table 2: Biodegradable analysis of Cs-Ti nanocomposites.
| Sample | Initial mass (gr) | Final mass (gr) | Mass. loss (%) |
| Cs | 0.05 | 0.02 | 60 |
| Cs-0.1 Ti | 0.1 | 0.05 | 50 |
| Cs-0.2 Ti | 0.1 | 0.05 | 50 |
| Cs-0.3 Ti | 0.14 | 0.09 | 35.71 |
| Cs-0.4 Ti | 0.15 | 0.10 | 33.33 |
| Cs-0.5 Ti | 0.17 | 0.12 | 29.41 |
Table 2 shows the decreasing mass of bioplastics and loss of mass after 2 months of hoarding process. Visually, the results of nanocomposites hoarded have changed, hoarded samples are brownish and appear to shrink even appear to be torn for sample without the addition of TiO2. This is clearly different from the initial conditions of bioplastics that are still smooth, clear for pure and white chitosan when TiO2 is added. The level of plastic biodegradation can be seen from the percentage mass loss of bioplastic material after being hoarded for a certain period.
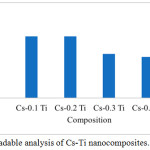 |
Figure 3: Biodegradable analysis of Cs-Ti nanocomposites. |
Figure 3 shows the addition of TiO2 affected the degradation process even though there was still a process of reduced mass. The decrease in mass tends to be long and slightly in line with the increasing number of TiO2 compositions added. This results in a smaller percentage of mass loss and shows that Cs-Ti nanocomposites took a long time to be completely degraded. It is due to the anti bacterial properties of Cs-Ti which causes the decomposition process of the nanocomposites to be inhibited.19
Antimicrobial Activity of Cs-Ti Nanocomposites
In a series of experiments, the antibacterial activities of chitosan and Ti-Cs nanocomposites were assessed using the S. aureus microbiological test system as described earlier. The results of this work can be seen at Table 3.
Table 3: Antimicrobial activity of Cs-Ti nanocomposites.
| Sample | UV Radiation | Without UV Radiation | |||
| CFU (mL-1) | Survival Ratio (%) | CFU (mL-1) | Survival Ratio (%) | ||
| Control | 2.9 x 105 | 100 | 2.9 x 105 | 100 | |
| Cs | 2 x 104 | 6.90 | 2 x 104 | 3.45 | |
| TiO2 | 0 | 0 | 0 | 0 | |
| Cs-0.1Ti | 0 | 0 | 0 | 0 | |
| Cs-0.2Ti | 0 | 0 | 0 | 0 | |
| Cs-0.3Ti | 0 | 0 | 0 | 0 | |
| Cs-0.4Ti | 0 | 0 | 0 | 0 | |
| Cs-0.5Ti | 0 | 0 | 0 | 0 | |
Table 3 shows the ability of Cs bioplastic to inhibit bacterial but it could not reduce S. aureus bacteria to zero. One of the antimicrobial properties of CS is a positively charged amino group that interacts with a negatively charged microbial cell membrane, which causes the destruction of proteins and intracellular constituents of microorganisms. Cs has been shown to be more effective against gram-negative bacteria than gram-positive bacteria.18
Table 3 shows the presence of TiO2 in Ti-Cs composite increasing the ability to kill bacteria to zero. The survival of S. aureus bacteria was significantly impaired due to the addition of TiO2 nanoparticles. CFU calculations performed after 24 hours of incubation under UV irradiation or without UV irradiation have shown similar results, namely successfully killing all S. aureus bacteria added. This study have in common results with the previous experiments conducted by other researchers.18,20
The iluminated TiO2 capable of killing bacteria entirely is because CS-Ti positively charged interacts with membranes of charged lipid bacteria negatively affecting cell permeability, blocking cell growth and survival thus causing bacterial death. Biological organisms are killed by various reactive species such as hydroxyl radicals, hydrogen peroxide, or superoxide, which are produced in the photocatalytic process of TiO2 nanoparticles. When a TiO2 nanoparticle is irradiated with UV it will experience the generation of electrons in the conduction band and form a hole (h+) in the valence band. Interaction of holes with water molecules will produce hydroxyl radicals •OH. Radical •OH is an oxidizing agent from organic compounds. From this photocatalysis process, reactive radical species •OH and •O2 can be released which are strong oxidative substances to degrade organic compounds from the composition of bacterial cell walls.21 When there was no UV irradiation but TiO2 addition is carried out, it shows that TiO2 nanoparticles can kill bacteria entirely, but it is not clear how the mechanism is. It can be assumed that the mechanism that occurs is the same as Ag which both have antibacterial activity because Ag has a positive charge that can interact with a negative charge on bacteria. So that without UV irradiation, TiO2 can also act as an antibacterial.
Conclusion
Bioplastics can be synthesized with a mixture of chitosan and TiO2 with the interaction between chitosan and TiO2 nanoparticles as seen in the FTIR test results. The surface morphology of chitosan-TiO2 nanocomposites showed the distribution of granules which indicated that 25 nm TiO2 nanoparticles were scattered in the matrix. Tensile strength and elongation decrease with the addition of TiO2 nanoparticles, Cs-0.1Ti was the best result compared to other variations of TiO2 addition. Cs-Ti nanocomposites has been proven to effectively kill Staphylococcus aureus bacteria entirely with or without UV irradiation. Bioplastics with the addition of TiO2 could inhibited biodegradable process.
Acknowledgements
The authors would like to thank to the Islamic Development Bank for financial assistance under IDB-UNTIRTA Research Grant project No. 593/UN43.9/PL/2018.
References
- Gautam, R.B. and Kumar, S., Development in nano-particle embedded biodegradable polymers for pacaking and storage of fruits and vegetables. Journal of food Research and Technology. 2015; 3(2): 43-61.
- Goudarzi, V., Shahabi-Ghahfarrokhi, I., Photo-producible and photo-degradable starch/TiO2 bionanocomposite as a food packaging material: Development and characterization. International Journal of Biological Macromolecules. 2018; 106: 661-669.
- Avérous, L., Biodegradable Multiphase Systems Based on Plasticized Starch: a review. Journal of Macromolecular Science – Part C Polymer Reviews. 2004; C4 (3): 231–274.
- Haldorai, Y and Shim J-J. Novel Chitosan-TiO2 Nanohybrid: Preparation, Characterization, Antibacterial, and Photocatalytic Properties. Polymer Composites. 2014; 35 (2): 327-333.
- Mazin C., Thanshif, A., Anandapadmanabhan, Ashfaq, Mujeeb, A., dan Lobo, A. G. Study on the Effect of Nano TiO2 on Mechanical Properties of Chitosan. IOSR Journal of Mechanical and Civil Engineering. 2015; 12(3 Ver. I): 48-54.
- Ostafińska, A., Mikeśová,J., Krejćíková,S., Nevoralová,M., Śturcová,A., Zhigunov, A., Michálková,D., Ślouf, M. Thermoplastic starch composites with TiO2 particles: Preparation, morphology, rheology and mechanical properties. International Journal of Biological Macromoleculs. 2017; 101:273-282.
- Mallakpour, S. and Madani, M. Effect of Functionalized TiO2 on Mechanical, Thermal and Swelling Properties of Chitosan-Based Nanocomposite Films. Polymer-Plastics Technology and Engineering. 2015; 54(10): 1035-1042.
- Othman, S. H. Bio-nanocomposite Materials for Food Packaging Applications: Types of Biopolymer and Nano-sized Filler. Agriculture and Agricultural Science Procedia. 2014; 2: 296–303.
- Azeredo, H.M.C.D., Nanocomposites for Food Packaging Applications. Food Research International. 2009; 42: 1240-1253.
- Azeredo, H.M.C.D., Mattoso, L.H.C. and McHugh, T.H., Nanocomposites in Food Packaging – A Review, in “Advances in Diverse Industrial Applications of Nanocomposites”. In: Reddy, B. (Ed.), InTech. Available from: http://www.intechopen.com/books/advances-in-diverse-industrial-applications-of-nanocomposites/nanocomposites-in-food-packaging-a-review.
- Kustiningsih I, Slamet, Purwanto WW, Synthesis of Titania Nanotubes and Titania Nanowires by Combination Sonication hydrothermal Treatment and their Photocatalytic Activity for Hydrogen Production. International Journal of Technology. 2014; 5: 133-141.
- Ismail, S., Lockman, Z., Kian, T.W., Formation and photoelectrochemical properties of TiO2 nanotubes arrays in fluowrinatedorganic electrolyte, Journal of Mechanical Engineering and Science. 2017; 11: 3129-3136.
- Kustiningsih, I., Sutinah, Stefitizky, M., Slamet, Purwanto, W.W., Optimization of TiO2 nanowires synthesis using hydrothermal method for hydrogen production. Journal of Mechanical Engineering and sciences. 2018; 12(3): 3876-3887.
- Alexander, F., AlMheiri, M., Dahal, P., Abed, J., Rajput, N.S., Aubry, C., Viegas, J., Jouiad, M., Water splitting TiO2 composite material based on black silicon as an efficient photocatalyst. Solar Energy Materials and Solar Celss. 2018; 180: 236-242.
- Sikander, U., Sufian, S., KuShaari, K., Chong, F.K., Effects of catalytic bed position oh hydrogen production by methane decomposition. Journal of Mechanical Engineering and Science. 2018; 12: 3313-3320.
- Li, B., Zhang, Y., Yang, Y., Qiu, W., Wang, X., Liu, B., Wang, Y., Sun, G., Synthesis, characterization, and antibacterial activity of chitosan/TiO2 nanocomposite against Xanthomonas oryzae pv. oryza. Carbohydrate Polymers. 2016; 152: 825-831.
- Li, Y., Zhang, W., Niu, J. F., & Chen, Y. S. (2012). Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano, 6,5164–5173.
- Visurraga, J. D., Meléndrez, M. F., García, A., Paulraj, M. & Cárdenas, G. 2010. Semitransparent Chitosan-TiO2 Nanotubes Composite Film for Food Package Applications. Journal of Applied Polymer Science, 116,3503-3515.
- Matsunaga, T., Tomoda, R., Nakajima, T., Wake, H. 1985. Photo-electrochemical Sterilization of Microbial Cells By Semiconductor Powders. FEMS Mikrobiol Lett 29(1-2): 211-214.
- Tsuang, Y.H., Sun, J.S., Huang, Y.C., Lu, C.H., Chang, W.H.S. and Wang, C.C. Studies of Photokilling of Bacteria Using Titanium Dioxide Nanoparticles. Artif Organs 2008; 32(2).
- Gumiero, M., Donatella P., Andrea P., Alessandro S., Lucilla L., Giuseppe C. and Rosanna T. Effect of TiO2 Photocatalytic Activity in a HDPE-based Food Packaging on the Structural and Microbiological Stability of a Short-ripened Cheese. Food Chemistry. 2013; 138(2-3): 1633-1640.

This work is licensed under a Creative Commons Attribution 4.0 International License.









