Characterisation of Biochar Obtained from Org./ Mat./ Drugs and Its Application for Removal of Ciprofloxacin
Department of Civil Engineering, SRM Institute of Science and Technology, Kattankulathur – 603202 , Tamil Nadu, India.
Corresponding Author E-mail: niki.maharathi1@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350323
Article Received on : 12-04-2019
Article Accepted on : 13-05-19
Article Published : 14 Jun 2019
Ciprofloxacin is an antibiotic compound that is used for various health issues like headaches, nervousness, nausea, vomiting etc. Ciprofloxacin is the second generation of quinolones in the different categories of antibiotics. After using this antibiotic, some percentages of the compound are not metabolized in the body and is excreted along with the urine and excreta. This will reach the treatment plant and the conventional treatment method is not designed to treat these micropollutants, so it is released into the environment. The presence of ciprofloxacin is detected in surface water samples collected from different areas of the world. This study is conducted to find an effective adsorbent that can remove the ciprofloxacin from wastewater. Biochar produced from agricultural waste is highly rich in carbon and made from the process called pyrolysis. Pyrolysis of biomass is carried out under lower temperatures and low oxygen content. Biochar is used to remove antibiotic compounds, naphthalene, and heavy metals. Biochar is economical and does not have any adverse effects on the environment. Biochar can be prepared from different types of organic biodegradable waste. Since, the quantity of municipal solid waste reaching the landfill site is increasing day by day, converting the organic waste into biochar can reduce the amount of waste reaching the landfill site. In this study, biochar prepared from rice husk at 300°C is the best adsorbent to remove ciprofloxacin from aqueous solution. The adsorption of ciprofloxacin is studied for various conditions. The samples were analyzed in the UV-Vis spectrophotometer and it shows good removal efficiency.
KEYWORDS:Adsorption; Biochar; Ciprofloxacin
Download this article as:| Copy the following to cite this article: Arun S, Maharathi P. Characterisation of Biochar Obtained from Org./ Mat./ Drugs and Its Application for Removal of Ciprofloxacin. Orient J Chem 2019;35(3). |
| Copy the following to cite this URL: Arun S, Maharathi P. Characterisation of Biochar Obtained from Org./ Mat./ Drugs and Its Application for Removal of Ciprofloxacin. Orient J Chem 2019;35(3). Available from: https://bit.ly/2RfR75D |
Introduction
Antibiotics are widely used in the treatment and prevention of infections. They may either kill or prevent the growth of bacteria. Ground and surface water get contaminated by pharmaceutical products which are an environmental problem causing widespread concern. Pharmaceutical products are discharged from private houses, hospitals, industries that reach municipal sewage treatment plants. These pharmaceutical products present in wastewater are not treated specifically in treatment plant, so they are not completely removed from wastewater and are directly allowed to discharge in to the environment.
The primary direction of the movement of the pharmaceuticals products into the environment is through human excretion and animal feeding operations. The industrial compounds are still present in wastewater after passing the wastewater treatment and enter through point discharge and surface runoff. Pharmaceutical compounds are the trace organic compounds present in the environment. The presence of trace organic compounds is having dangerous effects on wildlife and aquatic life. The antibiotics are present in drinking water sources that need to be examined. As microorganisms play an important role in wastewater treatment by biological processes for decomposing organic materials present in the wastewater, the trace pharmaceutical compounds present in the wastewater may cause death of microorganisms, hence it has to be removed.
Ciprofloxacin (CIP) is an antibiotic compound that is used for various health issues like headaches, nervousness, nausea, vomiting etc. Ciprofloxacin is the second generation of fluoroquinolone antibiotic. The presence of ciprofloxacin is not only contaminating the water sources but also increasing the risk to life. Ciprofloxacin is a common antibiotic prescribed by various medical practitioners for treatment of infections and those infections which are caused due to gram- negative bacteria. But it targets both gram positive and gram-negative bacteria. The main source of entry of ciprofloxacin in to the environment is incomplete metabolism by the body.10 Other sources are pharmaceutical industry effluent and surface runoff from landfill sites. Because of high solubility in water, occurrence of ciprofloxacin is reported in soil, surface water and wastewater all over the world.11 The amount of ciprofloxacin present in various sources of water is increasing as the demand of the antibiotic is increasing by the population which is produced by the pharmaceutical industry.
Biochar produced from agricultural waste which is highly rich in carbon is made from the process called pyrolysis and pyrolysis of biomass process is carried out under relatively higher temperatures. Biochar is used as a good adsorbent because of its large specific surface area, porous in nature, the presence of functional groups and mineral components. It removes various environmental pollutants present in soil and water. For enhancing soil mostly agricultural waste is converted into fertilizer which increase the fertility of the soil. The amount of solid waste generated from various sources can be reduced by utilizing the waste in the preparation of the biochar which can be helpful for the environment as it reduces the amount of waste deposited in the landfills and can be helpful to make the climate change less severe. Biochar is used to remove antibiotic compounds, Poly Aromatic Hydrocarbons, naphthalene and heavy metals.1 Biochar is economical and it is not having any adverse effects on the environment. The pyrolysis process is dependent on temperature and can be optimized in order to produce biochar or energy. Pyrolysis process depends on the pyrolytic temperature residence time, adsorbent size, and rate of heating.
The materials that are required for the preparation of biochar are easily available. Biochar as compared to other adsorbent material is a low-cost adsorbent. Adsorption capacities are more in biochar as compared to other adsorbents. The biochar can be prepared from any kind of waste organic material. Thus, the waste material can be converted into a useful product which reduces the load on the landfill.
The emerging contaminants can be removed from the wastewater by adsorption process which is considered as one of the green technologies. The adsorption process shows high removal efficiency with minimum waste generation. A large number of research works have been carried out across the globe to find a suitable adsorbent material which has maximum adsorption, less cost and less waste generation.1 The tea leaves were used for the preparation of biochar for the removal of ciprofloxacin as millions of tons of used tea leaves were wasted after use that causes a burden on the ecological environment. The used tea leaves were pyrolyzed at 450°C has an excellent absorption ability and 33% removal efficiency for ciprofloxacin.2
The cassava dregs were used for the preparation of biochar at three different temperature and the biochar prepared at higher temperatures had the more surface area and larger micropore volumes i.e. at 750°C. The biochar produced at 750°C had the highest sorption affinity of ciprofloxacin.3 Adsorption of fluroquinolone antibiotics and biochar prepared from sludge is a low -cost adsorbent material for controlling the pollution caused by fluroquinolone antibiotics.4 The ciprofloxacin removal from artificial wastewater with sawdust, was also studied earlier. The supernatant was analysed at a different wavelength and the removal efficiency is maximum at 277 nm.5
The biochar prepared from municipal sludge is a low -cost adsorbent material for controlling the pollution caused by fluroquinolone antibiotics. The removal efficiency by using sludge derived biochar is nearly about 76%.4 The biochar that was produced through the microwave pyrolysis was used for removal of silver from an aqueous solution. Biochar prepared from bio-solids is a promising method to minimize water contamination and biosolids can be effectively managed by this environmentally sustainable approach to re-use it in the future.6 The removal of ciprofloxacin using the biochar prepared from peels of grapefruit was also showed good results. Biochar prepared with chitosan which is another adsorbent used for ciprofloxacin removal. The efficiency of chitosan biochar hydrogen beads for ciprofloxacin removal is 35% and chitosan biochar hydrogen beads is an adsorbent which has proved to be an economical and sustainable adsorbent material.8
This study was conducted to find the effectiveness biochar to remove ciprofloxacin antibiotic from wastewater. The biochar was produced from the pyrolysis of rice husk which is a locally available waste material. The aim of the investigation was to identify an economical adsorbent for removing of ciprofloxacin from wastewater.
Methodology
Materials
Ciprofloxacin was purchased from Sigma Aldrich. 100 ppm solution was prepared by dissolving it in 4% buffer solution (NaOH). Rice husk was collected from rice mill, Chennai. Milli Q water was used in experimental procedure.
Biochar Preparation
The rice husk was collected and shredded into small pieces and then washed with acetone. The moisture content, ash content and volatile matter content of rice husk was measured by using ASTM methods. The rice husk was dried and required amount was taken in a crucible with tight fitted lid.The biochar was prepared at different temperature in a muffle furnace with a certain duration of time. After attaining the particular temperature, it was allowed to cool down and then removed from the furnace. To determine the effect of residence time, the biochar was removed from the muffle furnace at different times. The biochar was then stored in desiccators so that outside moisture doesn’t affect it. The biochar was prepared at four different temperatures that are 200°C, 250°C, 300°C and 350°C. But the biochar prepared at 350°C turned into ash. The biochar was then powdered and sieved to get uniform size for the adsorption experiments.
Adsorption Procedure
The stock solution was prepared by dissolving ciprofloxacin in 4% NaOH buffer solution and then 100 ppm standard solution was prepared from it.100 ppm ciprofloxacin solution was used for all adsorption experiments.
100 ppm of standard solution was spiked over 0.2 g of biochar to find the effect of shaking time on removal. The experiment was done for three different biochar prepared at 200°C, 250°C and 300°C. Four sets of the conical flask were placed in the shaker at 80rpm for different shaking time i.e. 45 minutes, 60 minutes, 90 minutes and 120 minutes.
100 ppm of standard solution was spiked over different concentration of biochar to find the effect of concentration of biochar on removal. The experiment was done for three different biochar prepared at 200°C, 250°C and 300°C with a constant shaking time.
100 ppm of standard solution was spiked over 0.2 g of biochar produced at 250°C, 200°C, 300°C in five different flasks to find the effect of residence time of biochar in the muffle furnace.
To find the effect of initial concentration of ciprofloxacin, 20 ppm, 40 ppm, 60 ppm, 80 ppm and 100 ppm solutions were spiked with optimum concentration of biochar and was shaken for optimum time.
Characterisation of Biochar
Biochar yield was found out by weighing the rice husk before and after biochar preparation. The SEM (FEI Quanta 200 F, Thermo Fisher scientific) images used to study the morphology of the biochar before and after adsorption. Presence of functional groups before and after adsorption were studied by using FTIR (IRT racer – 100, Shimadzu, Japan). The specific surface area and pore size were determined by BET (Smart sorb93, Smart instruments company, India) analysis.
Ciprofloxacin Analysis
After each experiment, the solution was placed in the micro-centrifuge tubes. All the tubes were centrifuged at 5000 rpm for 15 minutes which allowed the biochar to settle that was present in each of the sample. Then the samples were filtered and tested in UV-VIS Spectro-photometer at 285 nm. Removal efficiency was calculated by using the following equation
(C0 – Ce) × 100/ C0
where Ce and C0 are final and initial concentration of ciprofloxacin respectively. Amount of adsorption was calculated by using the equation (C0 – Ce) × v/w, where w is the weight of the adsorbent used and v is the sample volume.
Adsorption Kinetics and Isotherms
From the kinetic experiments it was found that biochar prepared at 300°C was showing best results. So, sorption kinetics of 300°C biochar was analysed with pseudo first order and pseudo second order model.
Freundlich and Langmuir isotherm models were used to analyse the results to find the best model for the sorption experiments.
Results and Discussion
Biochar preparation and adsorption experiments were carried out as per the procedure explained in the methodology. The biochar prepared at different pyrolysistemperature ie, 200°C, 250°C, 300°C were used for the experiments with variation in time, variation with concentration of biochar, variation with residence time in the muffle furnace and variation with initial concentration of the sorbent. The organic material was kept inside the muffle furnace for certain duration of time which is an important factor for the preparation of biochar. Biochar cannot be produced at a higher pyrolysistemperature from the organic material. The temperature should be less than 700°C for preparing biochar, depending on the characteristics of the material. The pyrolysistemperature always varies for the conversion of the biomass into biochar. For rice husk the maximum temperature was found to be 300°C, above this temperature the rice husk became ash. The efficiency of the adsorption of biochar is also affected by the pyrolysistemperature making it a significant factor in adsorption process. The capacity of adsorption increases as the surface area increases, which in turn depends on the temperature; with the increase in temperature, there is an increase in surface area. This depends on the type of feedstock material that is being used for the preparation of biochar.
Characterisation of Rice Husk
The moisture content, ash content and volatile matter content of rice husk is shown in table 1.
Table 1: characteristics of rice husk.
| feed stock | moisture content (%) | Ash content (%) | Volatile matter content (%) |
| rice husk | 11.2 | 14.7 | 79.8 |
The biochar yield of the rice husk biochar was found to be 78.5 %.
Effect of Removal Efficiency with Time
The adsorption efficiency of the rice husk was determined by using a UV Spectrophotometer. Ciprofloxacin adsorption experiment was carried out using the rice husk biochar that was prepared at 200°C, 250°C, and 300°C was shaken for different time i.e. 45 minutes, 60 minutes, 90 minutes and 120 minutes in orbital shaker.
The figure1 shows the the removal efficiency of ciprofloxacin with time.The removal efficiency increases with the temperature at which the biochar is prepared. The biochar that was produced at 200°C showed removal efficiency of ciprofloxacin which is around 50% at 48 minutes then it is decreasing with the increase of time. The biochar that was produced at 250°C showed a removal efficiency of around 85% at 90 minutes,and then it reaches equilibrium. The biochar that was produced at 300°C gave the removal efficiency of 96% at 90 minutes and became stable at 120 minutes.Hence the optimum time of removal is considred as 90 minutes. The biochar produced at 300°C is the best biochar which gave the highest removal efficiency.
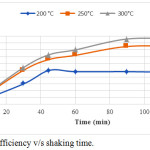 |
Figure 1: Removal efficiency v/s shaking time. |
Effect of Different Concentration of Biochar
As shown in figure 2, ciprofloxacin adsorption on rice husk biochar was minimum at 0.2g. Removal efficiency decreased with the decrease of the concentration of the biochar. The biochar produced from three different temperatures that are at 200°C, 250°C, and 300°C gave a maximum removal efficiency of 52%, 75% and 82% respectively. Since the optimum time obtained from the previous experiment is 90 minutes ,all the flasks were shaken for a constant shaking time ie, 90 minutes. The biochar prepared at 300°C is showing the maximum removal efficiency and the optimum biochar concentration is 0.6 g.
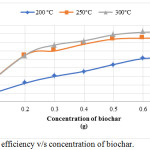 |
Figure 2: Removal efficiency v/s concentration of biochar. |
Effect of Different Pyrolytic Temperature with Different Residence Time Inside the Muffle Furnace
The residence time of biochar preparation also play an important role in the removal ciprofloxacin from the aqueous solution. Figure 3 shows the relation between removal and residence time. For all the three type of biochars the ciprofloxacin adsorption were maximum at 120 minutes. It indicates that the optimum residence time for biochar preparation is 120 minutes. The maximum removal efficiency of biochar produced at 200°C, 250°C and 300°C are 68%, 80%, and 82% respectively.
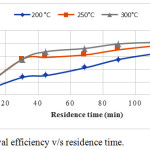 |
Figure 3: Removal efficiency v/s residence time. |
The optimum time and concentration of biochar for this experiment are 90 minutes and 0.6 g which was obtained from the previous experiments. This experiment also shows that biochar at 300°C is the best biochar for removing ciprofloxacin from aqueous solution.
Effect of Different Initial Concentration of Ciprofloxacin Solution
The experiment was done with 20 ppm, 40 ppm, 60 ppm 80 ppm and 100 ppm of ciprofloxacin solutions.The removal efficiency versus initial concentration of ciprofloxacin are shown in figure 4. For this experiments the optimum time was 90 minutes and optimum biochar concentration was 0.6 g Since the maximum removal efficiency of ciprofloxacin was obtained for biochar prepared at 300°C in the previous experiments, that biochar is only considered for this experiments. As the concentration of ciprofloxacin increases, the removal efficiency also increases. The results were also used to find the best fit in isotherm models.
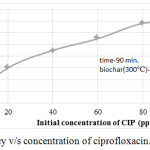 |
Figure 4: Removal efficiency v/s concentration of ciprofloxacin. |
Characterisation Results
The maximum removal efficiency of the ciprofloxacin is at 300°C at 90 minutes of shaking, so further analysis was carried out using the adsorption of ciprofloxacin with the above-mentioned temperature of biochar shaken with the sample. The characteristic functional group of rice husk biochar was identified by analysing the FT-IR spectra. The FT-IR spectra were inspected from 400 to 4000 cm-1 infrared spectral region of the rice husk biochar before and after adsorption are shown in figure 5. There are a greater number of peaks in infrared spectra of the rice husk biochar before adsorption. According to the graph, there is a broad peak around 3400 cm–¹, which shows the –OH functional groups including the stretching vibrations. The peaks at 2950 cm–¹ and 2850 cm–¹, shows symmetric and asymmetric stretching vibrations of Methylene,2 A small peak between 2500 cm–¹ and 2000 cm–¹ shows the presence of triple bonds. At 1600 cm–¹ the C=C bond in the aromatic ring, at 1500 cm–¹ the C-H alkanes and at 1050 cm-1 C-O in phenol are clearly identified.12 When compared to the infrared spectra after adsorption result, some of the peaks are formed and some peaks are disappeared from the spectra. The new peaks are at 3400 cm–¹, 1620 cm–¹ and 1050 cm–¹ and they are assigned to amino acids, hydroxyl group, stretching vibrations of amide group, phenolic C-H stretching vibrations, C=O stretching respectively. The sharp peak 1050 cm–¹ was reduced in FTIR image after adsorption which indicates there is a considerable reduction in C-O in phenol group. The peaks get flattened between 2500 cm–¹ and 2000 cm–¹ indicates the role of triple bond compounds in adsorption. In general, stretching vibration of -OH group, phenolic C-OH stretch, C-O in phenol, C-H stretching vibration and C=O stretching play a vital role in ciprofloxacin adsorption.
 |
Figure 5: FT-IR Result (a) before and (b) after adsorption. |
From BET analysis,the average pore diameter, the pore capacity and surface area of the biochar after adsorption were 4.76 nm, 0.018 cm3/g and 9.6 m2/g and before adsorption it was 4.23 nm, 0.010 cm3/g and 19.26 m2/g respectively. This is almost same as that tea leaves biochar.2 which also gives a removal efficiency around 80%.
Fig. 6 shows the SEM images of biochar before adsorption experiment and after adsorption experiment.The morphology of the biochar before and after adsorption was different. Some few tiny pores are seen on the surface of the biochar after adsorption as compared to the surface of the biochar prior to adsorption. As shown in figure 6(a) there are non-uniform pores. For rice husk after ciprofloxacin adsorption, a a number of rod-shaped particles appeared which shows active points of adsorption.
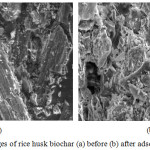 |
Figure 6: SEM images of rice husk biochar (a) before (b) after adsorption. |
Adsorption Kinetics
The kinetic studies conducted were correlated with two models, one is pseudo first order reaction model and the other one is pseudo second order reaction model. The pseudo first order model is shown in figure 7. Since the biochar prepared at 300°C is showing best results, kinetic studies were done only for this biochar.
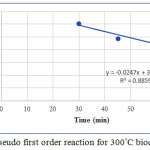 |
Figure 7: Pseudo first order reaction for 300°C biochar. |
The equation for pseudo first order reaction is given below ln (qe – qt) = ln qe – k1 t the equation for pseudo second order reaction is given below
![]()
Where qt is the ciprofloxacin adsorbed at time t and qe is the amount ciprofloxacin adsorbed at equilibrium. k1 and k2 are constants. The pseudo second order reaction model is shown in figure 8.
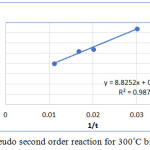 |
Figure 8: Pseudo second order reaction for 300°C biochar. |
From both the graphs it is clear that the best fit obtained is for pseudo second order reaction, because the R2 value obtained is 0.9874 for this model.
Adsorption Isotherms
Evenif the experiments were conducted for 200°C, 250°C, and 300°C biochar, the biochar prepared at 300°C was showing the good results in all aspects. So the isotherm studies were done only for 300°C biochar.The Langmuir model and the Freundlich model are the commonly used isotherm models to explain the nature of adsorption and adsorption capacity.
The Langmuir and Freundlich equations are shown below;
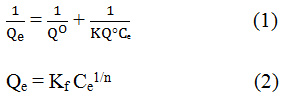
Where is the amount of CIP adsorbed per unit mass of adsorbent.
Ce is the CIP concentration in the solution at equilibrium,
Cf is the Freundlich coefficient,
n is the Freundlich constant,
K is the surface adsorption affinity constant,
Q0 is he adsorption capacity.
The Langmuir and Freundlich isotherms of the biochar prepared at 300°C are shown in figure 9 and figure 10.
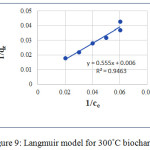 |
Figure 9: Langmuir model for 300°C biochar. |
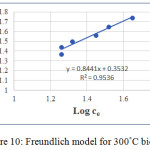 |
Figure 10: Freundlich model for 300°C biochar. |
According to above two models Freundlich isotherm is the best fit for explaining the adsorption capacity. The R2 value for this one is 0.9536. Fangze Li et al (2016) also explains the Freundlich isotherm is the best fit for adsorbing ciprofloxacin on biochar prepared from cassava drugs. 1/n value close to 1 indicates that isotherm is linear.
Conclusion
This experiment was conducted with an aim to find a cost-effective way to remove ciprofloxacin antibiotic that is contaminating the various water sources, since the water treatment plant and wastewater treatment plant is not treating the ciprofloxacin antibiotic specifically. The rice husk was used for the preparation of biochar and further experiment was done using this biochar for adsorption of ciprofloxacin antibiotic. The adsorption experiments reveal that rice husk biochar is very effective in removing the ciprofloxacin from the solution. The removal efficiency was found to be 96% at 90 min using the biochar produced at 300°C.Thus a waste material can be converted into a useful adsorbent with low cost.
Acknowledgements
The author acknowledge the support given by SRM research institute kattankulathur, Chennai.
Confilict of Interest
There is no confilict of interest.
References
- Daughton C.G Daughton and T.A. Ternes.;Pharmaceutical and Personal care Products in the Environment: Agents of subtle change, Environmental Health Perspectives, (1999), 107, 907-938.
- R.C. Yang, X, Flowers, H.S. Yang, X, Flowers, singer, P.C.; Occurrence and removal of pharmaceuticals and personal care products (PPCPs) in an advanced wastewater reclamation plant. Water Res.(2011) 45, 5218–5228.
- A Carmalin Sophia, Limab Eder C.;Removal of emerging contaminants from the environment by adsorption .Ecotoxicology and Environmental Safety 150 (2018) 1–17.
- Li Jie , Yu Guangwei , Pan Lanjia, Li Chunxing, You Futian, XieShengyu, WangYie, Ma Jianli, Shang Xiaofu.; Study of ciprofloxacin removal by biochar obtained from used tea leaves (2018), JES-01400.
- Li Fangze, Feng Dan, Deng Hui, Yu Huamei, Ge Chengjun.;Effects of biochars prepared from cassava dregs on sorption behaviour of ciprofloxacin. Procedia Environmental Sciences 31 (2016) 795 – 803.
- Yao Hong , Lu Jian , Wu Jun , Lu Zeyu ,Wilson P.Chris, Shen Yan.;Adsorption of Fluoroquinolone Antibiotics by Wastewater Sludge Biochar: Role of the Sludge Source. Water Air Soil Pollut (2013) 224:1370.
- Bajpai Sunil Kumar, Bajpai Manjula, and Rai Neelam.;Sorptive removal of ciprofloxacin hydrochloride from simulated wastewater using sawdust: Kinetic study and effect of pH (2016) ISSN 0378-4738.
- Elsa Antunes , Mohan V. Jacob , Graham Brodie , Philip A. Schneider.;Silver removal from aqueous solution by biochar produced from bio-solids via microwave pyrolysis .Journal of Environmental Management 203 (2017) 264e272.
- Afzal Muhammad Zaheer ,Sun Xue-Fei, Liu Jun, Song Chao, Wang Shu-Guang, Javed Asif.;Enhancement of ciprofloxacin sorption on chitosan/biochar hydrogel beads. Science of the Total Environment 639 (2018) 560–569.
- Y Sun Y., H Li, C Li.G.,Y Gao.B., Y Yue.Q., B Li.X.;Characterisation and ciprofloxacin adsorption properties of activated carbon prepared from biomass waste by H 3PO 4 activation, Bioresource technology (2016),217, 239-244.
- Fagbohungbe Michael O., Herbert Ben M.J., Hurst Lois , Ibeto Cynthia N., Li Hong, Usmani Shams Q., Semple Kirk T.;The challenges of anaerobic digestion and the role of biochar in optimizing anaerobic digestion. Waste Management (2016).
- Li Ronghua, Deng Hongxia , Zhang Xiaofeng , Wang Jim J., Kumar Mukesh Awasthi, Wang Quan , Xiao Ran , Zhou Baoyue , Du Juan, Zhang Zengqiang.; High-efficiency removal of Pb(II) and humate by a CeO2–MoS2 hybrid magnetic biochar. S0960-8524, 2018, 31, 485-8.

This work is licensed under a Creative Commons Attribution 4.0 International License.










