Antioxidant Potential of Pistacia Vera L. Fruit Hull, Anchusa Strigosa Flowers and Ilex Paraguariensis A. St.-Hil. leaves Extract
Ekbal Hasan Al-Khateeb*, Ghada Ahmad Al-Assi, Ashok K. Shakya, Naseer Al-Rawi and Naeem Shalan
Faculty of Pharmacy and Medical Sciences PO Box 263, Al-Ahliyya Amman University, Amman-19328, Jordan.
Corresponding Author E-mail: ekbal_7893@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/350309
Article Received on : 26-12-2018
Article Accepted on : 05-05-2019
Article Published : 10 May 2019
The macerated methanolic extract of P. vera L., A. strigosa and I. paraguariensis A. St.-Hil. were used for evaluating their antioxidant. The results of present study showed that the IC50 value for Pistacia vera L., Anchusa strigosa and Ilex paraguariensis A. St.-Hil. extracts were 5.85 ± 0.11, 43.75 ± 1.05 and 8.98 ± 0.65µg/ml respectively compared to 1.48 ± 0.05 µg/ml of ascorbic acid against DPPH radical. The IC50 values for β-carotene bleaching (BCB) assay for P. vera L., A. strigosa and I. paraguariensis A. St.-Hil. extracts were 390.1 ± 7.5, 425.8 ± 6.5 and 410.2 ± 9.0 µg/ml respectively compared to 9.5 ± 0.4 µg/ml of rutin. The results of β-carotene bleaching (BCB) assay showed that the plant extract exhibits weak activity compared to rutin. In conclusion, the present study indicates that these plants and their phytochemical constituents can be exploited in future extensively for controlling oxidative stress and ailments.
KEYWORDS:Antioxidant Potential; Β-Carotene Bleaching Assay; DPPH Radical Scavenging Activity; Natural Products; Oxidative Stress; Redox Properties
Download this article as:| Copy the following to cite this article: Al-Khateeb E. H, Al-Assi G. A, Shakya A. K, Al-Rawi N, Shalan N. Antioxidant Potential of Pistacia Vera L. Fruit Hull, Anchusa Strigosa Flowers and Ilex Paraguariensis A. St.-Hil. leaves Extract. Orient J Chem 2019;35(3). |
| Copy the following to cite this URL: Al-Khateeb E. H, Al-Assi G. A, Shakya A. K, Al-Rawi N, Shalan N. Antioxidant Potential of Pistacia Vera L. Fruit Hull, Anchusa Strigosa Flowers and Ilex Paraguariensis A. St.-Hil. leaves Extract. Orient J Chem 2019;35(3). Available from: https://bit.ly/308zZmt |
Introduction
Antioxidants are compounds that scavenge the free radicals, which are responsible for aging. Research studies suggest that free radicals are highly reactive, forms covalent bonds with certain enzymes and cause tissue damage.1 Natural products and phytochemicals, like flavonoid or polyphenolic compounds can scavenge the free radicals by their redox properties which allow them to act as reducing agents, hydrogen donor, chelator and singlet oxygen scavenger.2,3 These can prevent cell damage and aging process. Dietary antioxidants and supplements are responsible for protecting our system from many diseases (heart failure, hepato- and renal-toxicity) and progression of the disease.4,5
Pistacia vera L., is a plant member of Anacardiaceae family and native to Asia. Pistachio nut is mostly produced in Iran and some other countries.2 The chemical constituents of pistachios include fatty acids and flavonoids such as luteolin and vitamin-E (tocopherol), in addition to the presence of poly-phenols (catechins) which have been shown to be protective agents against cancer, cardio-vascular disease and hypolipodemic activity.6 Seifaddinipore et al. (2018) have reported the cytotoxic effect and anti-angiogenesis potential of pistachio hull7 against breast cancer cells.
Anchusa strigosa Banks et Sol. (Boraginaceae) is a perennial herb that is very common and localized in Jordan, Iraq and Palestine, it is known as hem-hem in Jordan.8,9 The active constituents are the alkaloids, pyrrolizidine, polyphenols, oil, proteins and aliphatic hydrocarbons. The flower herbal tea used as an analgesic, diuretic, sedative, diaphoretic and anti-arthritis.8-10
In South America, infusion of dried leaves of Ilex paraguariensis, is used for different medical purposes.11 The phytochemical constituents are mainly caffeine, phenolic acid and saponins. It shows a psychostimulant effect and used as an analeptic in Brazil. It is also used in the management of obesity and cardio-vascular problems.12-14 Methylxanthines, like caffeine, phenolic acids, and saponins are the main constituents of this plant. It is commonly used as everyday as a potential substituent for coffee in the South America. This encouraging information and research guided us to carry further research on the antioxidant potential of methanolic extracts of some plant’s parts, such as the hull (leathery cover) of Pistacia vera L. (pistachio) fruit, Anchusa strigosa flowers and Ilex paraguariensis A. St. Hil. (yerba mate) leaves.
Experimental
Materials and Methods
Ascorbic acid, methanol, 2,2-diphenyl-1-picrylhydrazyl (DPPH), β–carotene, rutin and other solvents were purchased from Sigma Aldrich, USA, and used without any further purification. The cold maceration technique was used for extractions.15
Identification and Herbarium
Plant material like hull (leathery cover) of fresh Pistacia vera L. (pistachio) fruit, flowers of Anchusa strigosa and Ilex paraguariensis A. St. Hil. (Yerba mate) leaves were collected from the same source during Dec. 2015 till Sep. 2017 and identified from As-Salt, Jordan, planted at the vicinity of Al-Ahliyya Amman University, Amman, Jordan. Herbarium specimens have been deposited in the Faculty of Pharmacy and Medical Sciences, Al-Ahliyya Amman University after identification by taxonomist Prof. Dawood Al-Eisawi, The University of Jordan, Amman, Jordan. The plant parts were immediately dried, crushed, grinded and stored at -5°C until required for the preparation of extracts.
Extraction15
The plants materials were extracted separately. Pistacia vera L. Plant material (30 gm) was macerated in 300 ml of methanol. Similarly, 10 g of yerba mate leaves in 150 ml and 10 g of Anchusa strigosa flowers in 500 ml of methanol were macerated. The plant material was soaked in methanol and kept for 48 hours. Thereafter, the extract was filtered and evaporated using rotatory evaporator. The dried extracts were kept under nitrogen environment till further use.
Standard and Sample Solutions
A stock solution of ascorbic acid (1000 µg/ml) in water was prepared. From this stock solution a working standard (100 µg/ml) by diluting the stock solution using water. A series of different standard solutions (0.5-10 µg/ml) of ascorbic acid were prepared for the evaluation of DPPH scavenging activity.
A stock solution of DPPH (0.010 w/v g%) was prepared in methanol and kept under nitrogen environment. A working solution of DPPH (0.002%) was prepared from the stock solution.
The different stock solutions (1 mg/ml) of plant extract were prepared by dissolving appropriate amount of extract from these plants. A series of solutions of plant extract (0.78-800 µg/ml) were prepared for the antioxidant activity in methanol.
The free radical scavenging activity of extract was measured by using reported method.16,17
DPPH Scavenging Activity
The plant extracts were analyzed for its free radical scavenging activity using DPPH according to the reported method.16,17 In the present study a methanolic solution of 0.002% DPPH was used. Briefly, 1 ml of plant extract of different concentration in methanol and 1 ml of methanolic solution of 2,2-diphenyl-1-picrylhydrazyl (DPPH) solution were mixed and vortexed for 1 min., and kept aside in dark for 30 min. The absorbance of solutions was measures at 517 nm using methanol as blank. The absorbance of 0.002% DPPH solution mixed with equal volume of methanol was recorded at the beginning of the experiment. DPPH radical scavenging activity was determined using given equation (1) and the IC50 was calculated.
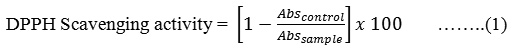
β-carotene Bleaching (BCB) Assay
β-carotene bleaching assay was conducted according to the earlier procedure.18 Briefly in 50 ml chloroform, 5 mg of β-carotene was dissolved. Forty milligrams of linoleic acid and 400 mg of Tween-20 were weighed and placed in an Erlenmeyer flask. Three ml aliquot of β-carotene prepared earlier in chloroform was added to it and mixed well. After 5 minutes, the chloroform was evaporated with the aid of nitrogen gas at 30°C. The resultant residue was re-dissolved in 100 ml of deionized water. The absorbance of this solution was measured at 470 and 700 nm.
Briefly 1 ml of β-carotene-linoleic acid emulsion was mixed with different solution of plant extract (3.13 µg/ml to 800 µg/ml) prepared earlier, capped and incubated at 50°C for 60 min. Appropriate control samples containing equivalent amount of methanol were prepared and used in the study. The absorbance of all samples and control solutions were recorded after 60 min at 470 and 700nm. Degradation rate (DR) and antioxidant activity was calculated using given formulas.
Degradation rate (DR) of β-carotene = Ln (Ainitial/Asample)/60 …….(2)
![]()
Statistical Analysis
Results are expressed as mean ± standard deviation (SD). GraphPad Prism 5 (San Diego, CA, USA) for Windows was used for statistical analyses of experimental data.
Results and Discussion
It is known that phytochemical reduce the risk of chronic diseases by scavenging the free radicals. Antioxidants appear to play a major role as protective agent for controlling the oxidative stress in diseased condition. Research indicates that the mechanism of different components depends on their structure and functional groups (poly phenolic compounds, flavonoids and related) responsible for scavenging these radicals. The extracts contain several chemical compounds which are collectively showing DPPH scavenging activity. Studies suggest that the free radical scavenging activity depends on type of phenolic compound as well as total phenolic content.
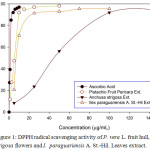 |
Figure 1: DPPH radical scavenging activity of P. vera L. fruit hull, A. strigosa flowers and I. paraguariensis A. St.-Hil. Leaves extract. |
The phenolic compounds scavenge free radicals through several proposed mechanisms, delocalization of electrons and formation of intramolecular hydrogen bonds are the proposed mechanism by which antioxidant scavenge the DPPH radical.
DPPH radical on reaction with antioxidant losses its free radical activity and the color of reagent changed to yellow from purple. Thereby decreasing the absorbance at 517 nm. The IC50 is calculated graphically by plotting the percent inhibition versus concentration of antioxidant. In the present study the IC50 value for Pistacia vera L., Anchusa strigosa and Ilex paraguariensis A. St.-Hil. extracts were 5.85±0.11, 43.75±1.05 and 8.98±0.65 µg/ml respectively compared to 1.48±0.05 µg/ml of ascorbic acid. The P. vera L. extract showed potent antioxidant which may be due to the phenolic compounds and flavonoids present in the extract. The results are presented here are comparable to the results reported by Özbek et al.19 IC50 value for P. vera L. hull extract prepared using 40% ethanol was 2.73 mg/ml. The antioxidant activity of I. paraguariensis leaves extract might be due to the presence of hydroxycinnamic acid derivatives and flavonols present.20 The antioxidant activity of these extracts were statistically comparable to the activity of the plant extract collected from different plant (viz. R. officianlis, P. harmala, T. polium, V. officinalis. A. herba-alba, and A. palaestinum) from Jordan and Palestine.21
The β-carotene bleaching (BCB) assay indicated the P. vera L., A. strigosa and I. paraguariensis A. St.-Hil. extracts were also capable of controlling the oxidation of β-carotene. The IC50 values for β-carotene bleaching (BCB) assay for P. vera L., A. strigosa and I. paraguariensis A. St.-Hil. extracts were 390.1±7.5, 425.8±6.5 and 410.2±9.0 µg/ml respectively compared to 9.5±0.4 µg/ml of rutin. The antioxidant investigations are reported in Table 1. Researchers earlier investigated antioxidant nature of phytochemicals such as catechins, gallocatechins, quercitin and rutin; caffeic, chlorogenic, sinapic, ferulic and p-coumaric acids.22
Table 1: DPPH radical scavenging activity and β-Carotene bleaching Assay of P. vera L. fruit hull, A. strigosa flower and, I. paraguariensis A. St.-Hil. Leaves extract.
| Samples |
IC50 (µg/ml) |
|
|
DPPH radical activity* |
β-Carotene bleaching Assay* |
|
| Pistacia vera L. hull Ext. |
5.85 ± 0.11 a |
390.1 ± 7.5 b |
| Anchusa strigosa flower Ext. |
43.75 ± 1.05 a |
425.8 ± 6.5 b, c |
| Ilex paraguariensis A. St.-Hil. leaves Ext. |
8.98 ± 0.65 a |
410.2 ± 9.0 b, c |
| Rutin |
– |
9.5 ± 0.4 |
| Ascorbic acid |
1.48 ± 0.05 |
– |
Values are given as mean ± SD (n=3),
a = Significantly less activity (p<0.05) as compared to reference compound ascorbic acid,
b= Significantly less activity (p<0.05) as compared to reference compound rutin,
c = difference between the activity of these extract is not significant (p>0.05).
Conclusion
The methanolic extract of P. vera L. and I. paraguariensis A. St.-Hil. extracts showed excellent antioxidant comparable to ascorbic acid. A. strigosa flower extract showed moderate activity against DPPH radical. The extract shows remarkable inhibition of β-carotene bleaching suggesting to study in detailed profile of phenolic compounds quantitatively to evaluate the antioxidant potential of these herbal plants used frequently and can be exploited in future extensively for controlling oxidative stress and ailments. As these plant extracts are having significant antioxidant activity hence these can also be exploited for the preservation of food, snack foods and beverages industry.
Acknowledgements
The authors wish to thank the Dean of Faculty of Pharmacy and Medical Sciences, Dean, Scientific Research, Al-Ahliyya Amman University, Amman, Jordan, for providing necessary facilities and financial assistance.
Conflicts of Interest
There is no conflicts of interest.
References
- Saeed, N.; Khan, M.R.; Shabbir, M. BMC Complementary and Alternative Medicine. 2012, 12(1), 221-.
- Hosseinzadeh, H.; Tabassi, S.A.; Moghadam, N.M.; Rashedinia, M.; Mehri, S.; Iranian Journal of Pharmaceutical Research: IJPR. 2012, 11(3), 879-887.
- Kähkönen, M.P.; Hopia, A.I., Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Journal of Agricultural and Food Chemistry. 1999, 47(10), 3954-62.
- Iqbal, J.; Zaib, S.; Farooq, U.; Khan, A.; Bibi, I.; Suleman, S. ISRN Pharmacology. 2012, Article ID 563267,6 pages, doi: 10.5402/2012/563267.563267
- Pandey, K.B.; Rizvi, S.I. Oxidative Medicine and Cellular Longivity, 2009, 2(5), 270–278.
- Bisignano, C.; Filocamo, A.; Faulks, R.M.; Mandalari, G. FEMS microbiology letters. 2013, 341(1), 62-7.
- Seifaddinipour, M.; Farghadani, R.; Namvar, F.; Mohamad, J.; Abdul Kadir, H. Molecules, 2018, 23(1), 110-125.
- Braca, A.; Fico, G.; Morelli, I.; De Simone, F.; Tomè, F; De Tommasi, N. Journal of Ethnopharmacology. 2003, 86(1), 63-7.
- Abbas, M.; Disi, A.; Al-Khalil, S. Jordan Journal of Pharmaceutical Sciences 2009; 2, 131-9.
- Al-Snafi, A.E. International Journal of Pharmacy and Pharmaceutical Sciences. 2014, 6(4), 7-10.
- Bastos, D.H.; Saldanha, L.A.; Catharino, R.R.; Sawaya, A.; Cunha, I.B.; Carvalho, P.O.; Eberlin, M.N. Molecules. 2007, 12(3), 423-32.
- Heck, C.I.; De Mejia, E.G. Journal of Food Science. 2007, 72(9), R138-51.
- Bastos, D.H.; Oliveira, D.D.; Matsumoto, R.T.; Carvalho, P.D.; Ribeiro, M.L. Medicinal and Aromatic Plant Science and Biotechnology, 2007; 1(1), 37-46.
- Bojić, M.; Simon Haas, V., Šarić, D.; Maleš, Ž. Journal of Analytical Methods in Chemistry. 2013; 2013: 658596, doi: 10.1155/2013/658596.
- Bandar, H.; Hijazi, A.; Rammal, H.; Hachem, A.; Saad, Z.; Badran, B. American Journal of Phytomedicine and Clinical Therapeutics, 2013, 1(6), 507-513.
- Naik, R.R.; Shakya, A.K.; Khalaf, N.A.; Abuhamdah, S.; Oriquat, G.A.; Maraqa, A. Jordan Journal of Pharmaceutical Sciences. 2015; 8(3), 181-193.
- Elagbar, Z.; Naik, R.R.; Shakya, A.K.; Bardaweel, S.K. Journal of Chemistry, 2016, 2016, 6 page. http://dx.doi.org/10.1155/2016/694809,
- El-Agbar, Z.A.; Naik, R.R.; Shakya, A.K. Oriental Journal of Chemistry. 2018, 34(3), 1368-74.
- Özbek, H. N., Halahlih, F., Göğüş, F., Yanık, D. K., & Azaizeh, H. Waste and Biomass Valorization, 2018, 1-10. https://doi.org/10.1007/s12649-018-0512-6.
- Mateos, R.; Baeza, G.; Sarriá, B.; Bravo, L. Food Chemistry, 2018, 241, 232-241.
- Khalaf, N.; Naik, R.R; Shakya, A.K.; Shalan. N.; Othman, A. Oriental Journal of Chemistry, 2015, 31, 1923-28.
- Jing, L. J.; Mohamed, M.; Rahmat, A.; Bakar, M. F. A. Journal of Medicinal Plants Research, 2010, 4(1), 027-032.

This work is licensed under a Creative Commons Attribution 4.0 International License.









