Theoretical Study of the Interconversions of Selected Isomers from C8H13+ System: A DFT level of Study
Joseph Lianbuanga*1 and Zodinpuia Pachuau2
and Zodinpuia Pachuau2
Department of Chemistry, Mizoram University, Aizawl -796004, Mizoram, India.
Corresponding Author E-mail: josephlbpachuau@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/350241
Article Received on : 25-01-2019
Article Accepted on : 30-03-2019
Article Published : 22 Apr 2019
The selected isomers of C8H13+ are calculated to predict its relative energies and their stability for each species at the levels of density functional theory (DFT) using 6-311G+dp basis set. We also attempted to predict the isomerisation or mechanistic pathways by locating the transition States through the interruption of diagonal matrix as confirmed by the imaginary Eigen value one. The isomers of the known and new isomers are revealed and analysed after optimisation. The geometrical analysis proposed that Iso- VII might be the most stable isomer which is also proved by heat of reactions analysis and the activation energy of all the inter conversions are compared in the energy profile diagram.
KEYWORDS:Bicyclo[5.1.0]Octane; Bicycle[4.2.0]Octane; Geometrical Analysis and Energy Profile Diagram; Heat of Reactions; Octahydropentalene
Download this article as:| Copy the following to cite this article: Lianbuanga J, Pachuau Z. Theoretical Study of the Interconversions of Selected Isomers from C8H13+ System: A DFT level of Study. Orient J Chem 2019;35(2). |
| Copy the following to cite this URL: Lianbuanga J, Pachuau Z. Theoretical Study of the Interconversions of Selected Isomers from C8H13+ System: A DFT level of Study. Orient J Chem 2019;35(2). Available from: https://bit.ly/2UMz38d |
Introduction
The inactive compounds of cyclo-octane have many importances in various fieldand are used for increasing the specific impulse in liquid propellant rocket engine and rocket power unit for realizing the same.1 And some active species like Bicyclo [4.2.0] Octane used in cardiovascular disorder like thrombosis, hypertension, atherosclerosis and inhibiting gastric acid secretion.2 There are seven (7) isomers under this system of study which are taken from C8H13+ system. All these isomers are under fused bicyclic rings which are- Bicyclo [5.1.0] octane, bicycle [4.2.0] octane and Octahydropentalene, because of the hydride shift and formation of new bond, we have two Isomers under bicyclo [5.1.0] octane- Iso-I and Iso-II, three isomers (Iso-III, Iso-IV and Iso-V) under bicycle [4.2.0] octane and Iso-VI and Iso-VII under Octahydropentalene. We predicted the inter conversion as to know the precursor for the preparation of these isomers. We have known that these isomers are the backbone of drugs and we can use this system for making required molecule.3
Methodology
The Isomers are modelled by Gauss View 5.0.8 and calculations are done by Gaussian09 Revision-A.02 on the NDDO (Neglect of differential diatomic overlap) integral approximation4 and it approaches to the class of zero differential Overlap (ZDO)methods where all the two electron integrals including two-centre charge distributions are excluded.5 A series of approximations are used to speed up calculations and a number of corrections are made to correct these approximate quantum model for all the isomers.6 The electron density which is a function of space and time is used in DFT; it also improved Hartree-Fock by treating the correlated motion of electrons.7 The exchange correlation energy is typically separated into the exchange part and correlation part especially in LDA. Here, Local Spin- density approximation (LSDA)is involved (Kumar 2008). The main aim is to minimize the energy of different isomers that can be obtained by optimization using density functional theory at B3LYP level of theory using 6-311G+(dp) basis set.8 The isomers obtained are arranged according to their stability orders.
Results and Discussion
All of these Isomers are fused bicyclic rings.9 Iso- I and Iso- II [Bicyclo( 5.1.0) Octane] are formed by a combination of cycloheptane and cyclopropane. Both Iso- III, Iso- IV and Iso-V are under Bicyclo[4.2.0] Octane but they are differed in cation positions. Octahydropentalene structure is seen in Iso-VI and Iso-VII.
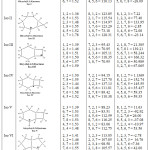 |
Table 1: Geometrical analysis of bond length, bond angle and dihedral angle. |
The bond length of Iso-I ranges between 1.43 A ô to 1.53 A ô and there is a double bond between carbon 3 and 2. The bond length of Iso-II has 1.36 A ô to 1.55 A ô and the cabocation is shifted to carbon 2, two double bonds are formed inside the isomer and it is arranged as chair form which gives more stability than the other isomers. Iso -III has a bond length ranges from 1.39 A ô to 1.59 A ô, the carbocation position at carbon 1 changes the bond length and breaking of bond between carbon & and 2 position and double bond is seen between 1 and 2. The shifting of hydrogen in Iso-IV formed a carbocation at 2 position, the bond length of Iso-IV ranges from 1.44 A ô to 1.58 A ô, the short and long distance of the carbons are due to the distortion of the rings, so Iso- V also ranges from 1.39 A ô to 1.53 A ô. The shortest distance is only because of the formation of double bond between 2 and 3. In Iso-VI, the ranges of bond lengths are 1.41 A ô to 1.55 A ô, the double bond environment of 2,3 and 1, 2 are 1.41 and 1.46 respectively. The carbocation is formed at 3 positions, the bond length of 2,3 and 3,4 are 1.46 each and the opposite side of the bond length between 6,7 and 7,8 are 1.54 each.
The highest bond angle is observed in the double bond carbon (2,3,4= 131.54) of Iso-I and it is made by cycloheptene and the smallest close ring cyclopropane on the other side, the whole isomer is a boat like structure and the orientation of Iso-II is Chair-Boat conformation and the new two double bonds caused distortion of the ring . Iso-III, Iso-IV and Iso-V are made by a combination of cyclohexane and cyclobutane (But the position of carbocation changes accordingly), the cyclobutane part is broken in all the isomers which give the different variety of bond angles inside and outside the ring as shown in table-I but among these isomer Iso-IV is supposed to have the most stable isomer because the carbocation is located at the common carbon at 2 position and their bond angle ranges from 70.95 to 118.35, 89.42 to 137.13 and 83.06 ô to 116.25 ô respectively as shown in the table- I. Same conditions are discernible in Iso-VI and Iso-VII, even though the structures are same as these isomers are made by two two cyclopentane, the positions of cations are different among these isomers so that we got the different ranges of bond angle. Cation is seen at 2 carbon in Iso- VI and at 3 carbon at Iso-VII. Iso-VII is the most stable isomer as we see from the three dimensional arrangement which can be proved by symmetrical nature of the bond angles (2,3,7 and 4,3,7 = 111.88 ô, 4,5,6 and 8,1,2 = 104.0 ô).
There are irregular dihedral angles in Iso-I because of the cation at carbon 3 inside cycloheptene ring, the dihedral angle ranges from -3.1546φ to 55.86. The 1,2 hydrite shift brought new orientation in Iso-II, there is a breaking of bond (between 1 and 7 carbons) and two new double bonds are formed ( between 1 and 2, 7 and 8 carbons) which give symmetrical arrangement among the carbons, the dihedral angles of 1,2,3,4 and 4,5,6,7 are 77.40φ and -69.97φ and their difference is only 8φ. Iso- III, Iso-IV and Iso – V are in the same class of bicyclo (4.2.0) Octane but they differ in the position of cations, the ranges of dihedral angles of Iso-III are 21.38φ to 166.32φ. Among these isomers, Iso- IV is supposed to be the most stable with the dihedral angle ranges from 1.13φ to 152.74φ which might be due to the position of cation at the common carbon 2. And Iso-V ranges between 17.47φ to 152.86φ but there is distortion of the molecule. Iso- VI and Iso – VII are in the same class of octahydropentalene and Iso-VII is more stable between them which is also the cation carbon exactly at the common carbon which caused less effect inside the whole isomer and the ranges of dihedral angles are 13.85φ to 176.20φ respectively.
Heat of Reaction
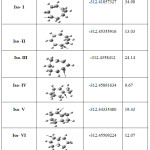 |
Table 2: Relative energies of the selected C8H13+ isomers at the level of B3LYP/6-311G+dp: |
The hartree energy ranges from -312.41857327 to -312.47432396 as in Table-II. Iso-VII (Octahydropentalene) is the highest hartree energy and is taken as a reference (0) (Lianbuanga et al. 2011), in this isomer, we have cyclopentane ring on both sides of the molecule and the cation nature is found at the center carbon which gdid not effect much inside the ring likewise we have similar structural formula in Iso-VI but different cation position that is the third most stable isomer and their difference is 12.07 Kcals/mol , the second most stable isomer is Iso-IV (9.67 Kcals/mol ) which is followed by Iso-III and Iso – IV, both have the same molecular formula with Iso-IV but differ in Cation position and their difference is 24.14 Kcals/mol and 19.43 Kcals/mol respectively with respect to Iso-VII Kcals/mol and the least stable isomer is Iso-I with a difference of 34.98 Kcals/mol as shown in Table-I. From this table, the order of stability are Iso-VII > Iso- IV >Iso-VI > Iso- II > Iso-V > Iso-III >Iso-I.
Characteristic Features of Transition
We have six transition states i.e. Ts-I, Ts-II, Ts-III, Ts-IV, Ts-V and Ts-VI which come under fused ring isomers only and their structures are shown in Table –III ( Calculated) and in Fig-I (Predicted). So the hartree energies can be compared after converted into Kcals/mol by studying three dimentional arrangement and from density functional method (DFT) observations. The order of energies as calculated by B3LYP/6-311G+ dp strategy is Ts-VI > Ts-II > Ts-IV > Ts-I > Ts-III> Ts-V as shown in Table – II. Here, the most stable transition states Ts-VI is found between Iso-VI to Iso-VII. All of their hartree values are compared and convert it into Kcals/mol with respect to the highest stable isomer ( Iso-VII). The highest relative energy (which is also the lowest stable transition state ) is found in Ts- V (between Iso-V to Iso-VI) and their difference is 55.58 Kcals/mol and. All of the transition states have one (1) imaginary frequency value. In the meantime, according to the activation energy barrier as calculated by activation energy of transition state to reactant, the order of transition state can be arrange as Ts-I > Ts-VI > Ts-II > Ts-III > Ts-IV > Ts-V.
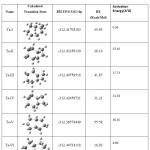 |
Table 3: Heat of formation and activation energy of the transition state as calculated by B3LYP/6-311G+dp level of theory. |
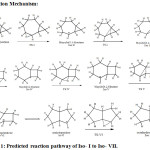 |
Figure 1: Predicted reaction pathway of Iso- I to Iso- VII. |
For the formation of Iso-II from Iso-I, we have 1,2- hydride shift passing through the activation energy barrier of 0.95 Kcals/mol which is supposed to be the fastest interconvertion in this reaction because it needs lowest energy to form the product Iso-II. In the conversion of Iso-II to Iso-III, there was bond breaking and new bond was form passing the barrier of 13.16 Kcals/mol to form the product. The cation position was changed and this undergo rearrangement to form bicyclo(4.2.0)octane Iso-III. In order to form Iso-IV from Iso-III, the activation energy of 17.73 Kcals/mol is required. From Iso-IV to Iso-V, we have 1,2-hydride shift with the activation energy of 21.54 Kcals/mol . This Iso-V breaks the bond and form new bond with the activation energy of 36.15 and forms Iso-VI, the newly formed isomer undergoes rearrangement to form octahydropentalene (Iso–VI). The formation of Iso-VII from Iso-VI was done by 2,3-hydride shift passing through the barrier of 4.86 Kcals/mol as shown in the potential energy Diagram ( Fig-II).
Energy Profile Diagram
The energy profile diagram of selected isomers from Iso- I to Iso –VII and Ts-I to Ts-VI are given as:
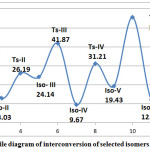 |
Figure 2: Energy profile diagram of interconversion of selected isomers of C8H13+. |
Conclusion
From the above reaction, the three isomers namely- Bicyclo (5.1.0) Octane, Bicyclo (4.2.0) octane and Octahydropentalene are involved in the interconversion
The Pople basis function with the mixing of d-orbital and p-orbital with diffused function (B3LYP/6-311G+dp) is used for the calculation, another level of calculation might be required for comparison for further studies.
From the calculation, the stability order can be arrange as Iso-VII > Iso- IV >Iso-VI > Iso- II > Iso-V > Iso-III >Iso-I.
The stability of the transition state with respect to the most stable isomer can be written as Ts-VI > Ts-II > Ts-IV > Ts-I > Ts-III> Ts-V
According to the activation energy barrier, the transition state can be arrange as follows – Ts-I > Ts-VI > Ts-II > Ts-III > Ts-IV > Ts-V.
The thermo chemistry and intrinsic reaction coordinate (IRC) calculations are needed to compare with these results for further studies.
Acknowledgements
The author thankfully to the University of Mizoram and Lunglei Government college for their scientific support.
Conflict of Interest
There is no conflict of interest.
References
- Ma C, Liu T, Yang L, Zu Y, Chen X, Zhang L, Zhang Y, Zhao C, Ionic liquid-based microwave-assisted extraction of essential oil and biphenyl cyclooctene lignans from Schisandra chinensis Baill fruits. Journal of chromatography, 2011, 1218, 8573-8580.
- Watson P, Coutsias A, Thompson A, Martin S, Topology of cyclo-octane energy landscape,. The journal of Chemical Physics, 2010, 132, 234115(1-6).
- Lewars E. G,Computational Chemistry. Introduction to the Theory and Applications of Molecular and Quantum Mechanics. Kluwer Academic Publishers, Dordrecht, 2010, 600-620.
- Almenningen A, Allinger L, Mastryukov S, Dorofeeva O, The Molecular structure and conformation of cyclo-octane as determined by electron diffraction and Molecular Mechanics Calculations. Journal of Phys. Chem, 1985, 89(2), 252-257.
- Kumar S. H,A Comparative QSPR Study of Alkanes with the Help of Computational Chemistry. Bull Korean Chem. Soc, 2008, 29, No-1: 67-76.
- Prasad R K, Quantum Chemistry (4th Edition), New Age International, New Delhi. 1996.
- Axel D. B, Perspective: Fifty years of density functional theory in chemical physics. The journal of chemical Physics, 2014, 140, 18A301.
- Joshua A.P, Dannenberg J.J, A comparison of the Behavior of Functional / Basis Set Combinations for Hydrogen-Bonding in the water Dimer with Emphasis on Basis Set Superposition Error. Journal of Computational Chemistry, 2011, 32, 1519–1527.
- Lianbuanga J., Pachuau Z. Employment of ab initio of Spiro, Fused, Bridged Head and Isolated Rings of Cyclo-octane Isomers: Their Relation with nature. Advances in Environmental Chemistry, AEC-2011, 260- 264.

This work is licensed under a Creative Commons Attribution 4.0 International License.









