Synthesis of Nanocurcumin - Alginate Conjugate and its Characterization by XRD, IR, UV-VIS Andraman Spectroscopy
1Sree Narayana College, Varkala, Kerala-695145, India.
2Sree Narayanacollege, Chengannoor, Kerala-689508, India.
3Sree Narayana College, Kollam, Kerala-691001, India.
Corresponding Author E-mail: jolysuresh@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350235
Article Received on : 13-06-2018
Article Accepted on : 06-03-2019
Article Published : 16 Apr 2019
The compounds which extracted from spices and herbs exhibit antiviral, anti-fungal and anti-cancerous effects having potential pharmacological uses. Curcumin, a component of turmeric, which has been used as a food additive and a coloring material in India and other Asian countries due to its potential medicinal properties. Earlier reports suggest that application of curcumin in foods is limited because of its low bioavailability and its high degradation in acid and alkaline medium. In this study the effect of stabilization of both free curcumin and nano curcumin – alginate conjugate in honey was studied by UV–vis absorption, IR, vibrational spectroscopy (Raman)and XRD. Curcumin is degraded in acid and alkaline medium is highly stable with the nano formulation. From this work it was deduced that in presence of surfactant honey curcumin- alginate inhibits the formation of small sub-products. This work reveals the complexation of curcumin with alginate in presence of surfactant honey was demonstrated to protect this molecule from the degradation. UV–vis, FTIR XRD and Raman spectroscopy were important to determine the nature of the structural modifications.
KEYWORDS:Curcumin; FTIR; UV-Vis; XRD and Raman Spectroscopy
Download this article as:| Copy the following to cite this article: Joly A, Latha M. S. Synthesis of Nanocurcumin - Alginate Conjugate and its Characterization by XRD, IR, UV-VIS Andraman Spectroscopy. Orient J Chem 2019;35(2). |
| Copy the following to cite this URL: Joly A, Latha M. S. Synthesis of Nanocurcumin - Alginate Conjugate and its Characterization by XRD, IR, UV-VIS Andraman Spectroscopy. Orient J Chem 2019;35(2). Available from: https://bit.ly/2v4kCx2 |
Introduction
Curcumin is a bright yellow chemical produced by plants. of the ginger family (Zingiberaceae). It is the principal curcuminoids in herbal supplement, cosmetics ingredient, food flavoring, and food colouring.1 Although curcumin has been used widely in Ayurvedic medicine, its potential medicinal properties are till unproven and are an area of active investigating field. Chemically, curcumin is a diarylheptanoid with two methoxide and hydroxyl groups, belonging to the group of curcuminoids, which are natural phenols responsible for turmeric’s yellow color. It is a tautomeric compound existing in enolic form in organic solvents and as a keto form in water (Figure-2).2
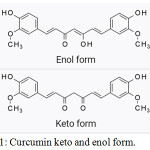 |
Figure 1: Curcumin keto and enol form. |
Moreover, evidences from long-term use process and preclinical trials have demonstrated low toxicity of curcumin, even at relatively high doses.3 However, it has been well known that the application of curcumin was limited due to its low water solubility, high degradation, and poor bioavailability.4-10 For decades, several studies have been made to compensate for these disadvantages, with the development of improved drug delivery methods.11-16 Recent researches witnessed the encouraging progress in the use of nanoscale drug delivery systems on curcumin such as loading curcumin into liposomes or nanoparticles, forming self-micro emulsifying drug delivery systems, cyclodextrin inclusions, and solid dispersions, as well as the latest reported technologies such as nano disks and nanotubes. However, good things never come easy. Applicational advancement of curcumin has been hindered by its water insolubility, degradation at alkaline pH, and photo degradation and thus extremely low bioavailability in both vascular and oral administration. Therefore, many inventions have been investigated, and an enhanced approach of using nanoscale drug delivery systems to overcome deficiencies. Among these methods, nanoscale drug delivery systems have become a source of concern for many researchers in the domain of tradional medicine.17 Many successful examples of the combination of nanotechnology and traditional medicine have been reported. Alginic acid is a natural hydrophilic gel with special physical-chemical and biological properties, including non-toxicity, chelating metal ions, biodegradability, antibacterial and antifungal effects, immune system stimulation, etching metal ions, biodegradability, antibacterial and antifungal effects, immune system stimulation, etc.18,19 In this study curcumin- alginate conjugate in honey as surfactant nanoparticles were synthesized by using the simple sonication green method and were characterized by SEM, TEM, Fourier Transform Infrared Spectroscopy (FTIR), Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) AND XRD.
Material and Methods
All chemicals were of reagent grade and were used without further purification. Distilled water was obtained from a Water Purifying System; acetic acid and NaOH were obtained from laboratory supplies (Tvpm) while curcumin powder and alginic acid were obtained from Merck.
Methods
About 2.5 × 10-4Mof curcumin was dissolved in 15mL of acetic acid solution containing t, followed by the addition of 5mL of 2% alginic acid and the mixture was stirred continuously for 1 h. After 1 h, 1mL of 1;1 surfactant solution (1% w/v) was added drop-wise to the mixture and stirred for an addition of 1h. Curcumin was loaded nano particles formed during the stirring. The curcumin loaded nanoparticles were collected by centrifugation and the samples were washed several times with ethanol to remove any excess of curcumin that adhered at the surface of the nanoparticles. This experiment is also repeated with NaOH solution.
Morphological Studies of Materials
Powder x-ray diffraction patterns were recorded on an X-ray diffraction (XRD; D8 Discover, Bruker AXS GmbH (λ = 1.5406 nm). X-ray Diffractometer (Bruker D8 Advance): X-ray diffractometer (Bruker D8), possess innovative design namely DAVICNI, which combines operating safety, ease of use and user safety. It can be operated in both Powder X-ray Diffraction (PXRD) and Grazing Incidence X-ray Diffraction (GIXRD) modes. Morphological analysis was examined on a Scanning Electron Microscope (SEM; JSM7600F, JEOL), Nanocurcumin solution prepared is evaporated to dryness and is ananlysed SEM by coating on the surface of carbon and sputtering with gold. The images of TEM were taken by a JEM-1010 (Jeol, Japan) operated at an accelerating voltage of 200 kV. Infrared data were examined on KBr pallets by using a Shimadzu IR Prestige-21 spectrometer (Japan. UV–vis and UV–vis-diffuse reflectance spectra were collected on a DR–Jasco V630 or a UV–vis DRS–Jasco V670 spectrophotometer that was equipped with a diffuse reflectance attachment in which BaSO4 was the reference. Micro Raman Spectrometer (with excitation wavelength of 514nm and 785nm available), Micro Raman Spectrometer (with excitation wavelength of 514nm and 785nm available). FT Raman Spectrometer is a multi-RAM stand alone model. The spectral range is 4000-50cm-1. About 50mg solid sample in liquid form is introduced in BRUKER RFS 27 stand-alone FT-Raman Spectrometer.
Results and Discussion
Morphology of Nanoparticle Curcumin by SEM and TEM
An SEM image of figure 2(a), (b), (c)) of NPs loaded with curcumin shows that particles are almost spherical in shape and not uniform. The particle size is in the range of 30-100nm. The SEM image of freshly prepared curcumin-micelles indicates that the self-assembled micelles are well dispersed as individual particles with spherical shape. This micrograph proves the incorporation of alginate inside the NPs matrix. Our results are consistent with those of other research. Morphological analysis was studied with electron microscopic images. In the SEM images of curcumin alginate nanoparticles, it can be seen clearly that the particles are aggregated, with approximate spherical shape having size80 –25 nm range (figure 1).
 |
Figure 2: (a), (b) and (c) SEM images of curcumin loaded alginate nanoparticles. |
The TEM images in figure 3- curcumin-alginate nanoparticles reveal that the nanoparticles are spherical in shape, and the average size was 20- 50 nm. Thus, the immobilized polymer on nanoparticles did not lead to the aggregation between the particles. This shows the appearance of the bonds on surface of nanoparticles
 |
Figure 3: Transmission Electron Microscopy of range 20 nm. |
Ultraviolet–Visible Spectroscopy Analysis
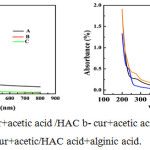 |
Figure 4: (a) and (b a-cur+acetic acid /HAC b- cur+acetic acid/HAC + alginic acid+honey c-cur+acetic/HAC acid+alginic acid. |
UV-Vis spectra (figure 4.1a) shows a broad absorption band at 285 nm in comparison with the two others such as figure 6.1(a)and(c). This may be due to the formation of nanocurcumin. The medium for this nanocurcumin formation is HAC.In HAC the nano curcumin formed is more stable than the nanocurcumin in NaOH. In NaOH the peak intensity is also less in comparison with the medium NaOH. In spectra (figure 4.1b) the peak intensity as well as broadening are greater for curcumin in HAC, but for curcumin alginate in surfactant has a less intense and much sharper peak. Formation of nanocurcumin shows the deviation of spectra. Figure 4.1 also shows the same variation in spectra.
Raman Spectroscopy for Analysis
Raman spectra (figure 5-2(a) and 2-(b)) reveals the nanocurcumin alginate in two different media in NaOH and in CH3COOH.In NaOH medium the curcumin under goes some degradation hence intensity reduces. Nano particles formed are found to be less compared to the nanoparticles formed from acetic acid. curcumin normal, a functional group vibration in the region of1500-1650 cm-1 is shown in the figure which is due to C=O vibration, but in the case of nano curcumin the peak at this region is very intense and sharp. Another major peak is at 1060 – 1150 cm-1. Raman spectra of nanocurcumin is too much intensive than normal curcumin.
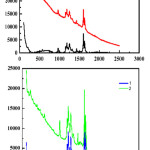 |
Figure 5: (a) and (b)) a-Raman 1 normal curcumin poly 8 Smooth and – b Raman 2 nano form. |
FTIR Analysis
The study of Infrared spectra (Figure-6) gives an idea about macro and nanoparticles of curcumin. Nanocurcumin has an intense and sharp peaks compared curcumin which have broad and less intense peaks. Curcumin nanoparticles have a stretching frequency near 1690cm-1 similar to the value of carbonyl frequency in curcumin and another stretching frequency value at 3000-3300 region which is of broad and of H-bonded –OH group in curcumin. The nanoparticles a less intense peaks in the region 950-750 cm−1 is due to aromatic C=C stretching. Next we have done a comparative study of nanocurcumin in different media in HAC and in NaOH. This also proves the stability and nanocurcumin formation of nanocurcumin. FTIR was studied to confirm the functional groups of the curcumin conjugate nanoparticles. The spectra of pure alginate, honey, nano curcumin alginate complex in honey–alginate conjugate( in NaOH and in HAC) were shown in figure 3. The presence of two strong absorption bands of all materials at around 3100-3400cm -1 and 1650-1700cm−1 shows the formation of nanoparticles. Moreover, the sharp intense peak band at 1690 cm−1 was confirmed as the strong stretching vibration of the two C=O functional group structure. The absorption bands at 1500-1400 cm−1, attributed to C-O groups in ether. Intense broad peaks around 3400-3100cm−1 is due to the O-H stretching of the nanoparticles. A less intense peaks in the region950-750 cm−1 is due to aromatic C=C stretching. The very less intense and very broad peaks at 1510 cm−1 and the band at 1627 cm−1 is attributed to the C–C stretching vibration of the aromatic ring. On the other hand, the appearance of two strong bands at about 13000 and 1040 cm−1 was confirmed as vibration of methyl phenyl ether group (C-O-CH3). This suggests that curcumin binds very strongly with alginate nanoparticles.
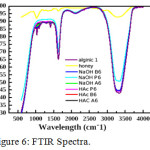 |
Figure 6: FTIR Spectra. |
XRD Analysis
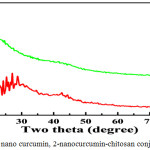 |
Figure 7: 1-Normal nano curcumin, 2-nanocurcumin-chitosan conjugate in honey. Click here to view figure |
Figure reveals the results of x-ray diffraction (XRD) analysis for curcumin conjugate nanoparticles synthesized by simple sonication method. The diffraction patterns have seven main peaks at 2θ values bet ween 15.2-30 corresponding to the reflection from different crystal planes. Positions and relative intensities of all the peaks are in similar to crystalline system of curcumin nanoparticles. The narrow shape peaks of the substance indicate that the nanoparticles have relatively very good crystalline state corresponding to the diffraction peaks of (110), (104) at 2θ positions of. Broadness of the diffraction peaks was related to particle sizes. Scherer’s equation was used to calculate the average particle size D. In this equation θ is the angle of the peak, β is the full width at half maximum (FWHM) of the respective XRD peak, λ is the x-ray radiation wave-length in angstroms, and k is a constant. The broadening of Bragg’s peaks indicates the formation of nanoparticles. The calculated mean crystallite size curcumin conjugate nanoparticles synthesized by the method 30-100 nm range. Further studies are needed for detailed XRD analysis.
![]()
In Vitro Cytotoxicity Assay
The cytotoxicity of free Cur and curcumin-alginate conjugate nanoparticles evaluated against A549 cell lines with the concentrations ranging from 6.25 μg ml−1 to 100 μg ml−1 is presented in table 1. It was observed that cell inhibition was decreased at lower concentrations of nanoparticles. That is due to the lower concentrations of drug loaded into nanoparticles. The cancer cells treated with a lower concentration (10 μg ml−1) of the curcumin loaded alginate nanoparticles show a low percentage of inhibitions (4.98%) whereas curcumin loaded alginate nanoparticles with higher concentrations (100 μg ml−1) have a very high ability to inhibit cancer cells (78.55 μg ml−1). With this concentration, the free-curcumin inhibited cancer cells were 94.4%. Because of the slower release rates of curcumin from nanoparticles, it reduces the interaction of the drug with a cell from the obtained results, table Curve software was used to calculate the IC50 values. IC50 is the drug concentration at which the drug includes 50% inhibition of the growth of the cells. The results obtained from the two samples of free-curcumin with an IC50 value of 73.03 and 11.37 μM ml−1, respectively. It suggests that curcumin n and curcuminloaded alginate nanoparticle shown the activity on cancer the A549 cell line n loaded alginate nanoparticles have no cytotoxicity wall.
Table 1: Cytotoxicity assay of free-curcumin and curcumin loaded alginate nanoparticles.
| A549 cells concentration µg/ml | Free curcumin (% of inhibition) | Curcumin-alginate nanoparticles |
| 6.25 | 11.47 | 4.98 |
| 12.5 | 68.26 | 16.39 |
| 25 | 85.5 | 24.32 |
| 50 | 91.4 | 31.59 |
| 100 | 94.4 | 78.55 |
| IC5012 | 11.37 | 73.03 |
Conclusion
Curcumin-alginate nanoparticles are synthesized by simple sonication method. The synthesized nanoparticles (NPs) showed relatively small size and good responsivity. The alginate-curcumins prepared were characterized by XRD, FTIR, SEM, TEM, and UV-Vis techniques. TEM results showed that the c nanoparticles have almost spherical shaped morphology and the average size from 18 to 50 nm. The FTIR data indicate that the functional groups were successfully attached on surface of the nanoparticles. The outcomes of these results showed that these nanoparticles are stable and highly soluble. The in vitro studies of Cur-loaded alginate showed up to 70% of adsorbed drug released in the buffer solution after 2800 min The in vitro release profile suggests that Cur-loaded alginate is promising in drug delivery carriers in cancer therapy.
References
- L. Li.; F.S. Braiteh.; R. Kurzroc.;, Liposome- encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis, cancer 104. 2005, 1322.
- N.K Narayanan.; D. Nargi.; C. Randolph.; B.A. Narayanan.; Liposomes encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mic., Int. J. cancer. 2009,125,1.
- Y. Sun.; Ch-Ch Lee.; W.-Ch Hung.; F.Y Chen.; M.T Lee.; H.W. Huang. The bound states of amphipathic drugs in lipid layers: study of curcumin, Biophys.Jour. 2008, 95, 2318.
- M.Garcia.; Jemal,; E.M Ward.; M.M Center.; Y. Hao.; R.L Siegel.; M.J. Thun.; Global cancer Facts & Figures ,American Cancer Society, Atlanta, GA. 2007,.1-46.
- A. Jemal.; R. Siegel.; E. Ward.; Y. Hao.; J. Xu.; M.J. Thun.; Cancer. J. Clin., 2009, 59,225.
- R. Autorino,; G.Di. Lorenzo.; R. Damiano.; S. De. Placido.; M.D’Armiento.; Urol. Int. 709200301. E.D. Crawford, Urology. 2005, 65,2.
- S. Urakami.; H. Shiina.; M. Sumura.; S. Honda.; K. Wake.; T. Hiraoka.; S. Inoue.; N. shikawa.; M. Igawa.; Int. Urol. Nephrol. 2008, 40,365.
- Raft. M.C.; Nature, 1992, 356, 397-400.
- Kerr J.F.R.; Wyllie. A.H.; and Currie. A.R.; (1972) Br. J. Cancer,1972,26, 239-257.
- Barry, M.A.; Reynolds. J.E. and Eastman. A.; Cancer Res. 1993 53, 2349-2357.
- Walton.M.I.; Whysong. D.; O.Connor. P.M.; Hockenbery. D.; Korsmeyer. S.J.; Kohn. K.W.; Cancer Res. 1993 53, 1853-1861.
- Satoskar, R.R. Shah, SJ. and Shenoy, S.G. (1986) Int. J.Clin.
- Satyajit Mondal, Soumen Ghosh : The effect of on the binding of cationic, anionic , nonionic surfactants with myoglobin (2017)1134.
- M. N. V. Ravi Kumar.; R. A. A. Muzzarelli.; C. Muzzarelli.; H. Sashiwa.; and A. J. Domb.; Chitosan Chemistry and Pharmaceutical Perspectives., Chem. Rev., 2004, 104 (12), 6017–6084.
- Gye Hwa Shin.; Seoung Kyun Chung.; Jun Tae Kim.; Hee Joung Joung.; Hyun Jin Park Joung and Hyun Jin Pak, Preparation of Chitosan – Coated Nanoliposomes for Improving the Mucoadhesive Property of Curcumin Using the Ethanol Injection Method: J. Agric. Food Chem. , 2013, 61 (46), pp 11119–111262013,61(46).
- Rajesh K.; Gangwar Geetanjali B.; Tomar Vinayak A.; Dhumale Smita Zinjarde.; Rishi. B.Sharma.; Suwarna Datar.; Curcumin Conjugated Silica nanoparticles for Improving Bioavailability and Its Anticancer Applications: Jour. of Agri. and Food Chem. 2013 61(40), 9632-9637.
- A Kunwar.; A. Barik.; R. Pandey.; ak.i.; Priyadarsini.; Transport of liposomal and albumin loaded curcumin to living cells:an absorption and fluorescence spectroscopic study, Biochem. Biophys. Acta Gen. subj. 2013, 27; 537(2):192-7.17609200601513.
- Maria Luisa Ruiz del Castilloa.; Eduardo López-Tobarb.; Santiago Sanchez-Cortesb.; Gema Floresa, Gracia Patricia Blancha Stabilization of curcumin against photodegradation by encapsulation in gamma-cyclodextrin: A study based on chromatographic and spectroscopic Raman and UV–visible and Vibrational Spectroscopy. Vibrational Spectroscopy 2015,81,106–111.
- Liang. Shen.; Hong-FangJi.; Theoretical study on physicochemical properties of curcumin. Spectrochimica Acta Part A .2007, 67 , 619–623.

This work is licensed under a Creative Commons Attribution 4.0 International License.










