Synthesis, Identification, and Infrared Spectroscopy Study of the Influence of Reaction Parameters Variations on Cu(C4O4).2H2O Synthesis
Louiza Zenkhri*1 , Salah Zenkhri2, Ahmed Boutarfaia3, Manal Bassa1, Aziza Sedrati1 and Khalida Benouna1
, Salah Zenkhri2, Ahmed Boutarfaia3, Manal Bassa1, Aziza Sedrati1 and Khalida Benouna1
1Univ Kasdi Merbah Ouargla, Department of Chemistry, Faculty of Mathematics and Matter Sciences, BP 511 Route Ghardaia, Ouargla,30 000- Algeria.
2Ecosystem Protection in Arid and Semi- Arid laboratory, Kasdi Merbah University, BP 511, 30000 Ouargla, Algeria.
3Univ Mohammed Khider Biskra, Science of Matter Department, Faculty of Exact Sciences and Sciences of Nature and life, BP 145 RP، 00700, Biskra.
Corresponding Author E-mail: louizazenkhri@yahoo.fr
DOI : http://dx.doi.org/10.13005/ojc/350248
Article Received on : 03-12-2018
Article Accepted on : 30-03-2019
Article Published : 11 Mar 2019
In this paper, we report synthesis, identification and study of different spectral measurements for Cu(C4O4).2H2O complex, in order to valorize effect of experimental conditions on resulting product nature. The structure was identified from a powder X rays diffraction. This complex was prepared for the first by Christian Rubel and Armin Weiss (1986). Comparative study for variation of different reaction parameters effect was interpreted by infrared spectrum observation for each product. In conclusion, we have put in evidence contribution effect of variation in temperature, pH, concentration, solvent, time of reaction on nature modification of the resulting product.
KEYWORDS:Cyclobutanetetrone; Hybrid Material; IR Spectroscopie; Reaction Parameter; X Rays Diffraction
Download this article as:| Copy the following to cite this article: Zenkhri L, Zenkhri S, Boutarfaia A, Bassa M, Sedrati A, Benouna K. Synthesis, Identification, and Infrared Spectroscopy Study of the Influence of Reaction Parameters Variations on Cu(C4O4).2H2O Synthesis. Orient J Chem 2019;35(2). |
| Copy the following to cite this URL: Zenkhri L, Zenkhri S, Boutarfaia A, Bassa M, Sedrati A, Benouna K. Synthesis, Identification, and Infrared Spectroscopy Study of the Influence of Reaction Parameters Variations on Cu(C4O4).2H2O Synthesis. Orient J Chem 2019;35(2). Available from: https://bit.ly/2NXRnoq |
Introduction
Several transition metal squarate tetrahydrate salts, M(C4O4).2H2O with divalent metal ions are known. Their structures consist of one dimensional metal squarate chains interlinked by hydrogen bonding.1 Contrary to earlier expectations, based on similar compounds formed with the oxocarbon ligand. The descriptive study of the crystalline structure of the compound Cu(C4O4).2H2O was carried out using the Mercry 3.8 program, based on the results of X-ray diffraction obtained in 1986 by the study of Christian Robel,2 on the base of the crystalline data file (cif). The asymmetric unit consists of a Cu copper atom, 4 carbon atoms, 6 oxygen atoms and 4 hydrogen atoms. The structure is three-dimensional. Structural metal units SBU (CuO6) bound to squarate anions formed by infinite layers parallel to the ab plane.
Materials and Methods
All chemicals used were analytical reagents and commercially purchased. IR spectra were obtained with FTIR-8300 CH-HMADZU spectrometer using KBr pellets in the 4000–400 cm. Elemental analysis for Cu, C, and O were performed using a MEB-EDX Quanta TM microanalyser. Diffraction powder experiments were performed at room temperature on a D8 Advence X-ray spectrometer. The sample for measurement was prepared by depositing the dried powder on a PMMA sample holder. The structure was identified using X’Pert High Score Plus program. The crystal lattice search was carried out with the Dicvol 06 program.3,4 The program used for powder pattern simulation and molecular graphics is a mercury program. The crystallographic data file (cif) number used is 1152266.
Synthesis Of Cu(C4O4).2H2O
A solution of squarique acid dihydrate (0,05702 g) in water (15 ml) was added dropwise with stirring at 50°C to a solution of Cu(NO3)3.H2O (0.2416 g) in distilled water (15 ml). The solution immediately became yellow suspension and was stirred for 4 h at room temperature. Then green clear solution (pH = 0,87) is formed and was stirred for a day and then cooled to room temperature. Green powder that formed was filtered and washed with water and dried in air (Fig. 1(a)).
Results and Discussion
Elementary Analysis
Scanning optical microscope analysis results show that the sample consists of three basic elements: carbon (30.69%), oxygen 43.08 %, copper (26.23%), with apparent morphology as very small crystallite (Fig. 1 (b)).
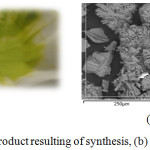 |
Figure 1: (a) Geen product resulting of synthesis, (b) Product morphology. |
X-Ray Diffraction (XRD)
The chemical formula of title compound was determined using X-ray powder diffraction technique. Figure 2 shows the curve recorded by the D8 Advance Difractometer in the examined angular range, which is usually the sample fingerprint. XRD experimental data of the synthesized product agree with simulated diffractogram with mercury 3.8 software for Cu(C4O4).2H2O complex (Rubel and Weiss 1986).2 A parts of experimental powder diffraction pattern of Cu(C4O4).2H2O (red) and powder pattern calculated from the atomic coordinates of cupper related compound (blue) are also presented with part of Rietveld plot which highlights the fabrication of the molecule of the same formula Cu(C4O4).2H2O (Fig. 2).
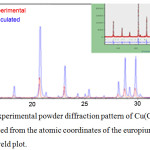 |
Figure 2: Parts of the experimental powder diffraction pattern of Cu(C4O4).2H2O (red) and the powder pattern calculated from the atomic coordinates of the europium related compound black with part of Rietveld plot. |
Indexing with DIVCOL 06 program leads in a monoclinic unit cell with parameters noted in table 1 and figures of merit M (21) = 86.0, F (21) = 129.9 (0.0027, 95) revealing crystallization quality. These results also show that the structure of this compound will be modeled in a space group different from that found in the work of Christian Rubel, because of different arrangement of Cell dimensions in the unit cell, as shown in the table 2.
Table 1: Indexing data of x-ray diffraction pattern for Cu(C4O4).2H2O synthesized.
| Crystal system | Monoclinic | |||||||
| Cell dimensions | a=6.2971 b=11.8307 c= 7.8321 β=100.247 V=574.18 | |||||||
| H | K | L | DOBS | DCAL | DOBS-DCAL | 2TH.OBS | 2TH.CAL | DIF.2TH. |
| 1 | 1 | 0 | 6.48374 | 6.48444 | 0.00070- | 13.646 | 13.645 | 0.001 |
| 0 | 1 | 1 | 5.50871 | 5.50848 | 0.00023 | 16.076 | 16.077 | 0.001- |
| 1 | 1 | -1 | 4.86255 | 4.86152 | 0.00103 | 18.23 | 18.234 | 0.004- |
| 0 | 2 | 1 | 4.18191 | 4.29037 | 0.00018- | 20.687 | 20.686 | 0.001 |
| 1 | 1 | 1 | 3.96459 | 4.18232 | 0.00041- | 21.229 | 21.227 | 0.002 |
| 1 | 2 | -1 | 3.86213 | 3.96252 | 0.00207 | 22.407 | 22.419 | 0.012- |
| 2 | 0 | 0 | 3.67171 | 3.86296 | 0.00084- | 23.01 | 23.005 | 0.005 |
| 2 | 1 | 0 | 3.56892 | 3.67256 | 0.00085- | 24.22 | 24.215 | 0.006 |
| 1 | 2 | 1 | 3.51803 | 3.56793 | 0.001 | 24.929 | 24.936 | 0.007- |
| 1 | 3 | 0 | 3.51803 | 3.51843 | -0.0004 | 25.296 | 25.293 | 0.003 |
| 1 | 3 | -1 | 3.17284 | 3.51843 | 0.00001 | 28.101 | 28.101 | 0 |
| 0 | 0 | 2 | 3.10399 | 3.17282 | 0.00001 | 28.101 | 28.101 | 0 |
| 2 | 2 | -1 | 3.06132 | 0.06204 | 0.00072 | 29.147 | 29.14 | 0.007 |
| 0 | 1 | 2 | 3.00269 | 3.00283 | 0.00014 | 29.729 | 29.728 | 0.001 |
| 0 | 4 | 0 | 2.96287 | 2.96311 | 0.00024- | 30.138 | 30.136 | 0.003 |
| 1 | 3 | 1 | / | 2.95903 | 0.00384 | / | 30.178 | 0.040- |
| 1 | 4 | -1 | 2.58815 | 2.58832 | -0.00017 | 34.63 | 34.628 | 0.002 |
| 3 | 1 | 0 | 2.51498 | 2.51441 | 0.00056 | 35.671 | 35.679 | 0.008- |
| 1 | 2 | 2 | 2.46897 | 2.46941 | -0.00044 | 36.359 | 36.352 | 0.007 |
| 1 | 4 | 1 | / | 2.46822 | 0.00074 | / | 36.37 | 0.011- |
| 3 | 2 | 0 | 2.35015 | 2.35958 | 0.00006 | 38.107 | 38.108 | -0.001 |
| 2 | 4 | 0 | 2.35015 | 2.34957 | 0.00059 | 38.266 | 38.276 | -0.01 |
| 1 | 5 | 0 | 2.26487 | 2.26508 | -0.00021 | 39.767 | 39.763 | 0.004 |
| NUMBER OF LINE | Q> = 0.19211.10-04 | |||||||
| LINES INPUT = 21 | FIGURES OF MERIT | |||||||
| LINES INDEXED = 21 | 1.- M( 21) = 86.0 | |||||||
| LINES CALCULATED = 59 | 2.- F( 21) = 129.9(0.0027، 59) | |||||||
Table 2: Cell parameters of Cu(C4O4).2H2O.
| Experimental result | 6.2971 | 11.8307 | 7.8321 | 90 | 100.247 | 90 | 574.18 | In this work | |
| Calculated result | P21/C | 7.818 | 11.816 | 6.295 | 90 | 100.3 | 90 | 572.45 | (Rubel and Weiss1986) |
Cristal Structure
The compound described previously in the literature.2 The central Cu atom has octahedral coordination geometry composed of six oxygen atoms, tree from squarate ligands and tree from water molecule (Fig. 3).
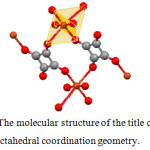 |
Figure 3: The molecular structure of the title compound, showing octahedral coordination geometry. |
Infrared Spectroscopy Analysis
Once the product crystalline structure is known, and for purpose of optimize reactions model for this material preparation, we propose the registration of an infrared spectrum template for this compound and then carried out similar chemical reactions with modification of parameters reaction for every time. The study of reaction parameters influence on products nature was described by interpretation of the recorded infrared spectra.
Figure 4 shows the infrared spectrum recorded for Cu(C4O4).2H2O in the spectral range (4000-400) cm-1 frequency range at room temperature.
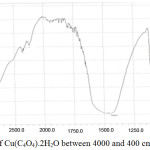 |
Figure 4: FT-Infrared of Cu(C4O4).2H2O between 4000 and 400 cm-1. |
The IR spectra exhibit the characteristic bands of both squarate and bands of cupper-oxygen. The spectrum shows that most of the bands observed are broad and dense, especially those corresponding to the OH group, followed by intermediate bands, weak, less dense, and representing the other functional groups in the sample. In the table 3, are listed the values of the waves of the functional groups in cm-1 with the type of vibration.
Table 3: Characteristic infrared bands of Cu(C4O4).2H2O and their interpretations.
| Band in this work (cm-1) | Band (Cm-1) in | |
| υC-C | 1095.5 | (Reinprecht and al, 1980) |
| υC-O | 1446.5-1500 | (Bellamy, 1975) |
| υC=O, υC=C | 1750 | 1822 (Baglin and Rose, 1970) |
| υO-H | 3031.9-3278 | (Erer et al, 2010) |
| υCu-O | 466.7 – 644.2 | (Doreswamy et al,,2005) |
The spectrum shows a weak and less dense peak at 1095.5 Cm-1 corresponds to C-C bond vibrations in squaric acid, while the relatively broad and dense band 1446.5 Cm-1 represents CO bond vibration in the same anion. The band 3031.7 Cm-1 represents the vibration of the OH bond of the water molecules. Metal-oxygen (Cu-O) bond expressed by the very low peak 466.7 cm-1 and Cu-H2O bond is represented by the peak 644.2 cm-1. These values are in agreement with the results of previous research.9
Effect of Changing Reaction Factors on Compound Formation
To study Effect of changing reaction factors on compound formation, we performed a series of five experiments, modifying each time one of the conditions associated with the experimental factors. We obtained results as mentioned in Table 4.
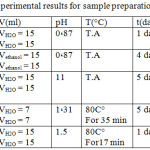 |
Table 4: Conditions and experimental results for sample preparation. |
Most of the samples were prepared at room temperature. Table 4 results shows that the high temperature did not affect final product nature, whereas color of product change. It is noted that the interaction of copper nitrate and squaric acid in the presence of NaOH (pH = 11) increases the yield of the reaction relative to the other samples. Duration of the interactions was short and in all cases we obtained samples in powder form.
To determine modification effect of reaction factors on the experimental product of previous series of reactions, we recorded FTIR spectra of five remaining samples (Fig. 5) and compared them template spectrum. All spectra were almost identical in terms of spectrum but marked by significant displacements. However, we note that same group can lead to several types of vibrations and therefore to absorption at different frequencies and explains these different experimental conditions from one sample to another.
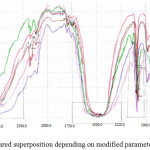 |
Figure 5: FT-Infrared superposition depending on modified parameter of the reaction. |
The comparison of the IR spectra in the highest frequency, related to the O-Hindice elongation motions, does not show significant difference between the four crystalline forms with the exception of the sample noted product 3.The product 2 coded spectrum shows an increase in band width and density, indicating the presence of impurities in the sample and an increase in alcoholic (OH) function, this can be justified by the nature of the solvent use (ethanol).
Raising the reaction temperature from ambient to 80°C does not affect the nature of the final product. The reaction time was short and we obtained the samples in powder form in all cases. With regard to the effect of pH, the reaction carried out at pH = 11.04 represented by the spectra coded product 3, the width of the band has been decreased with the position centered at about 3384.8 cm-1 and the emergence of a new band at 1125cm-1. In the lower frequency region small energy differences could be observed, although the form of the bands is identical in exption for product 3 and 5 at 898.8 cm-1. In the domain (644-4.66) cm-1 those band belonging to Cu-O.
Conclusion
In this research, we presented the synthesis, identification and study of the reactivity of a chemical compound of formula CuC4O4.2H2O using physico-chemical analysis techniques to determine its quantitative and qualitative composition and describe its crystalline structure. Using the X-ray diffraction technique, we have defined the vibrations of the functional group by infrared radiation, in order to be able to give a spectrum to the study of variation of the reaction parameters on the nature of the product.
We concluded similarity in the spectrum when we change the temperature, concentration and time of the reaction, so their general structure is similar. The modification of the pH and of the solvent leads to the increase of alcoholic function and the presence of impurities in the product.
Acknowledgments
This research was supported by the Algerian Ministry higher education and scientific research (Project PROFAS B). we majuscule thank the CRAPC center for helpful discussion, and the facility to use his experimental equipment.
References
- Frankenbach, G. M.; Beno, M. A.; Kini, A. M.; Williams, J. M.; Welp, U.; Thompson, J. E.; Inorg Chim Actu., 1992, 192, 195-200.
- Robl, C.; Weiss.; Z. Naturforsch., 1986, 41b, 1341-1345.
- Boultif, A.; Louer, D.; J. Appl. Cryst., 2004, 37, 724-73.
- Louer, D.; Louer, M.; J. Appl. Cryst. 1972, 5, 271-275.
- James, T.; Reinprecht, J. G.; Miller, G. C.; Vogel, M. S.; HADDAD, H.; Inorganic Chemistry. 1980, 19, 374-385.
- Bellamy, L. J.; Chapman and Hall. 1975, I.
- Baglin, F.G.; Rose, C.B.; Spectrochim. Acta. 1970, 26A, 2293.
- Erer, H.; Yesilel, O.Z.; Büyükgüngör, O. Polyhedron. 2010, 29, 1163-1167.
- Doreswamy, B. H.; Mahendra, M.; Sridhar, M. A.; Prasada, S. J.; Varughese, P. A.; George, J.; Varghese, G.; Materials Letters. 2005, 59, 1206-1213.

This work is licensed under a Creative Commons Attribution 4.0 International License.









