Synthesis and Antimicrobial Activity of Some 1-Arylideneamino-4-(4-Chlorobenzylidene)-2-Methyl-1H-Imidazolin-5(4H)-One Compounds
Cong Tien Nguyen*1 , Dao Thi Hong Dinh1, Thin Van Nguyen2, Giang Duc Le2 and Hien Cao Nguyen3
, Dao Thi Hong Dinh1, Thin Van Nguyen2, Giang Duc Le2 and Hien Cao Nguyen3
1Faculty of Chemistry, Ho Chi Minh City University of Education, 280 An Duong Vuong St., District 5, 700000, Ho Chi Minh city, Vietnam.
2School of Natural Sciences Education, Vinh University, 182 Le Duan St., 460000, Vinh city, Vietnam.
3Department of Chemical Technology, Ho Chi Minh City University of Food Industry, 140 Le Trong Tan St., Tan Phu District, 700000, Ho Chi Minh city, Vietnam.
Corresponding Author E-mail: congnt@hcmue.edu.vn
DOI : http://dx.doi.org/10.13005/ojc/350245
Article Received on : 09-08-2018
Article Accepted on : 17-03-2019
Article Published : 20 Apr 2019
4-Chlorobenzylidene-2-methyl-(4H)-oxazol-5-one, which were prepared from 4-chlorobenzaldehyde and acetylglycine in reaction with hydrazine hydrate in ethanol gave 1-amino-4-(4-chlorobenzylidene)-2-methyl-1H-imidazolin-5(4H)-one. However, treatment of 4-chlorobenzylidene-2-methyl-(4H)-oxazol-5-one with hydrazine hydrate in pyridine yielded 3-(4-chlorophenyl)propanohydrazide. Reaction of 1-amino-4-(4-chlorobenzylidene)-2-methyl-1H-imidazolin-5(4H)-one with aromatic aldehydes gave eight corresponding Schiff’s bases namely 1-arylideneamino-4-(4-chlorobenzylidene)-2-methyl-1H-imidazolin-5(4H)-ones. The structure of the 3-(4-chlorophenyl)propanohydrazide and the imidazoline-5-one compounds was confirmed by IR, 1H-NMR and MS spectral data. The Schiff’s bases were tested for antimicrobial activities against several strains of Gram-positive, Gram-negative bacteria, molds and yeasts.
KEYWORDS:Antimicrobial Activities; 1-Amino-4-(4-Chlorobenzylidene)-2-Methyl-1H-Imidazolin-5(4H)-One; 4-Chlorobenzylidene-2-Methyl-(4H)-Oxazol-5-One; Schiff Bases; Spectral Data
Download this article as:| Copy the following to cite this article: Nguyen C. T, Dinh D. T. H, Nguyen T. H, Le G. D, Nguyen H. C. Synthesis and Antimicrobial Activity of Some 1-Arylideneamino-4-(4-Chlorobenzylidene)-2-Methyl-1H-Imidazolin-5(4H)-One Compounds. Orient J Chem 2019;35(2). |
| Copy the following to cite this URL: Nguyen C. T, Dinh D. T. H, Nguyen T. H, Le G. D, Nguyen H. C. Synthesis and Antimicrobial Activity of Some 1-Arylideneamino-4-(4-Chlorobenzylidene)-2-Methyl-1H-Imidazolin-5(4H)-One Compounds. Orient J Chem 2019;35(2). Available from: https://bit.ly/2GvBu6d |
Introduction
The imidazole nucleus has proven to be unusually fertile source of medicinal agents such as anticancer agents, antioxidant agents, antifungal agents and antimicrobial agents.1,2,3
Schiff’s bases have been shown as biologically versatile compounds having anti-inflammatory, anticonvulsant, antimalarial, antiviral, antifungal and antibacterial.4,5 Recently, some Schiff’s bases containing imidazole nucleus have been reported to possess various biological properties like antioxidant and antimicrobial activity.6,7 To contribute to the study of the synthesis and properties of the Schiff’s bases, we report here the preparation of Schiff’s bases containing imidazolin-5-one moiety starting from 4-chlorobenzaldehyde and N-acetylglycine via 4-chlorobenzylidene-2-methyl-(4H)-oxazol-5-one first and then 1-amino-4-(4-chlorobenzylidene)-2-methyl-1H-imidazolin-5(4H)-one. Next, the antimicrobial activities of the synthesized Schiff’s bases were investigated. Besides that, in the present work, the transformations of the oxazole compound in solvents such as ethanol and pyridine were also mentioned.
Material and Methods
All reagents are of commercial quality and used without any further purification.
Melting points were measured in open capillary tubes on a Gallenkamp melting point apparatus and are uncorrected. The IR spectra of synthesized compounds were recorded on FTIR–8400S–SHIMADZU spectrometer using KBr pellets. 1H-NMR was recorded on Bruker Avance spectrometer at 500 MHz using a DMSO-d6 as a solvent and tetramethylsilane (TMS) as an internal standard, as well as the 13C-NMR, HSQC, HMBC spectra were recorded at 125 MHz. The MS spectra were recorded on a Bruker micrOTOF–Q 10187 spectrometer.
The synthesis of the Schiff’s bases containing 2-methylimidazolin-5-one heterocycles were performed following the steps shown in Scheme 1.
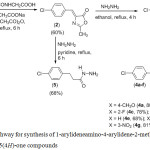 |
Scheme 1: Pathway for synthesis of 1-arylideneamino-4-arylidene-2-methyl-1H-imidazolin-5(4H)-one compounds. |
Synthesis of 4-(4-Chlorobenzylidene)-2-Methyloxazol-5(4H)-one (2)
Oxazole (2) was synthesized according to the procedure reported previously8,9 An equimolar mixture of 4-chlorobenzaldehyde (7.03 g, 0.05 mol) and acetylglycine (5.85 g, 0.05 mol) in freshly distilled acetic anhydride (25 mL) containing fused anhydrous sodium acetate (4.1 g) was refluxed for 3 h and then cooled. The solid was triturated with cold saturated solution of sodium carbonate and filtered, washed with water, air dried and recrystallized from ethanol. Yield 60%, mp. 158-160°C; IR (ν, cm-1): 1800, 1772, 1661, 1605; 1H-NMR (δ, ppm and J, Hz): 8.19 (2H, d, 3J = 8.0, ArH), 7.57 (2H, d, 3J = 8.0, ArH), 7.52 (2H, d, 3J = 8.5, ArH), 7.23 (1H, s, ClC6H4CH=) and 2.39 (3H, s, CH3).
Synthesis of 1-Amino-4-(4-Chlorobenzylidene)-2-Methyl-1H-Imidazolin-5-one (3)
To a solution of compound (2) (2.22 g, 0.0l mole) in 50 ml of absolute ethanol, hydrazine hydrate 50% (7.5 mL, 0.03 mole) was added and the reaction mixture was refluxed for 4.0 hours. On cooling, the formed precipitate was filtered off and crystallized from ethanol. Yield 56 %, mp. 192-193°C; IR (ν, cm-1): 3318, 3209 (N-H), 1705, 1643 (C=O, C=N, C=C); 1H-NMR (δ, ppm and J, Hz): 8.22 (2H, d, 3J = 8.5, Ar-H), 7.50 (2H, d, 3J = 8.5, Ar-H), 6.98 (1H, s, ClC6H4CH=), 5.11 (2H, s, NH2) and 2.31 (3H, s, CH3); 13C-NMR: 168.8, 166.0 (C=O, C=N), 137.8, 134.4, 133.4, 133.0, 128.7, 123.2 (Car and Colefilic) and 14.5 (CH3).
General Procedure for Synthesis of 1-Arylideneamino-4-(Chlorobenzylidene)-2-Methyl-1H-Imidazolin-5(4H)-one Compounds (4a-h)
An equimolar quantity of imidazolin-5-one (3) and a definite aldehyde was refluxed in ethanol for 6.0 hours. The reaction mixture was cooled down to room temperature and the precipitate obtained was filtered off and crystallized from dioxane to give the corresponding products. As the result, yellow crystals are obtained for all cases. Yields, physical properties and IR spectral data of these Schiff’s bases were shown in Table 1. 1H-NMR and 13C-NMR data of the synthesized Schiff’s bases were summarized in Table 2 and 3, respectively.
Synthesis of 3-(4-chlorophenyl)propanohydrazide (5)
To a solution of compound (2) (2.22 g, 0.0l mole) in 30 ml of pyridine, hydrazine hydrate 50% (7.5 mL, 0.03 mole) was added and the reaction mixture was refluxed for 6.0 hours. On cooling, the formed precipitate was filtered off and crystallized from ethanol. Yield 68%, mp. 161-162°C; IR (ν, cm-1): 3379, 3302 and 3179 (N–H), 2924, 2855 (C–Haliphatic), ~1700 (C=O); 1H-NMR: 8.94 (1H, s, N-H), 7.31 (2H, d, 3J = 8.5, Ar-H), 7.21 (2H, d, 3J = 8.5, Ar-H), 4.15 (2H, br, NH2), 2.80 (2H, t, 3J = 7.5, CH2CO), and 2.31 (2H, t, 3J = 7.5, ArCH2); 13C-NMR: 170.5 (C=O), 140.2, 130.5, 130.1, 128.2 (Car) and 34.8, 30.2 (Caliphatic).
Antimicrobial Activity Assay
Antimicrobial activity of test samples was assayed using microbroth dilution methods of McKane, L., and Kandel.12 This means, the serial dilution technique was performed using 96-well microplates to determine the minimum inhibitory concentration (MIC) of the samples against test microorganisms, including Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 25923), Bacillus subtilis (ATCC 11774), Staphylococcus aureus subsp. (ATCC 11632), Aspergillus niger (439), Fusarium oxysporum (M 42), Saccharomyces cerevisiae (SH 20) and Candida albicans (ATCC 7754). Briefly, the activated microorganisms were diluted with the growth medium broth (Eugon Broth (Difco, USA) for bacteria, Mycophil (Difco, USA) for fungi) to give a turbidity equivalent to the McFarland 0.5 standard and used for susceptibility testing. The plates were covered and incubated overnight at 37°C for bacteria and at 30°C for 48 h for fungi. The lowest concentration which inhibited visible growth of test microorganisms was regarded as MIC. Proper controls were kept for each experiment.
Results and Discussion
The most important method for the synthesis of 1-aminoimidazolin-5-one compounds may be treatment of oxazolones with hydrazine. Therefore, as shown in Scheme 1, to obtain 1-amino-4-(4-chlorobenzylidene)-2-methyl-1H-imidazolin-5(4H)-one, hydrazine hydrate was treated with 4-(4-chlorobenzylidene)-2-methyloxazol-5(4H)-one (2), which was prepared by Erlenmeyer condensation of acetyl glycine with 4-chlorobenzaldehyde in presence of sodium acetate and acetic anhydride.
The synthesis of 4-(4-chlorobenzylidene)-2-methyloxazol-5(4H)-one (2) from 4-chlorobenzaldehyde and acetyl glycine was carried out according to the method described in our earlier work.8,9 The structure of the synthesized compound was conformable to its spectral data. According to the literatures, aminoimidazolin-5-one may be afforded by refluxing mixture of oxazolone and hydrazine in pyridine.10,11 However, the product yielded in the reaction of 4-(4-chlorobenzylidene)-2-methyloxazol-5(4H)-one (2) with hydrazine in pyridine was not desired 1-amino-4-(4-chlorobenzylidene)-2-methyl-1H-imidazolin-5(4H)-one compound. Mass spectrum of the product showed [M+H]+ ion peak at m/z 199.0684 which is not agreement with the molecular formula of the C11H10ClN3O (M = 235.0512) of 1-amino-4-(4-chlorobenzylidene)-2-methyl-1H-imidazolin-5(4H)-one. The 1H-NMR spectrum show the appearance of two adjacent methylene groups as two triplet signals at 2.79 and 2.31 ppm with intensity of 2H for each signals. The aromatic area appeared two doublet signals, both with intensity of 2H. Besides that, there are two broad signals, one at 8.94 ppm with intensity of 1H and one at 4.15 ppm with intensity of 2H corresponding with protons of the hydrazine group (-NHNH2). The 13C-NMR spectrum show the appearance of a signal of carbonyl carbon at 170.5 ppm, four signals of aromatic carbons at 128.2-140.2 ppm and two signals of aliphatic carbons at 30.2-34.8 ppm. All data showed that the product is 3-(4-chlorophenyl)propanohydrazide (C9H11ClN2O, M = 198.0560).
In the our previous work,8,9 the reaction of oxazole (2) with hydrazine hydrate occurs at room temperature to give 2-(acetamido)-3-(4-chlorophenyl)acrylohydrazide easily.8,9 Thus, we assume that after the formation of the hydrazide, this compound was transformed into N-(3-(4-chlorophenyl)-1-hydrazinyl-1-oxopropan-2-ylidene)acetamide, which was reduced by hydrazine to form 3-(4-chlorophenyl)propanohydrazide as description in the Figure 1.
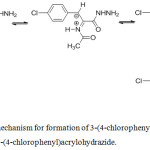 |
Figure 1: Proposed mechanism for formation of 3-(4-chlorophenyl)propanohydrazide from 2-(acetamido)-3-(4-chlorophenyl)acrylohydrazide. |
Changing the condition of reaction, we found out that 1-amino-4-(4-chlorobenzylidene)-2-methyl-1H-imidazolin-5(4H)-one compound (3) may be formed by refluxing 4-(4-chlorobenzylidene)-2-methyloxazol-5(4H)-one with hydrazine hydrate in ethanol. The structure of the product was first confirmed via its IR spectral data in which the presence of a characteristic absorption band around 3201-3456 cm-1 and a medium one around 1622-1647 cm-1 was in turn represented for N-H bonds and C=O group. Beside signal of the amino group at d 5.11 ppm (2H, singlet), signals of benzene ring (two doublet with J = 8.5 Hz at d 8.22 ppm and d 7.50 ppm), signal of olefinic proton (1H, singlet) at d 6.98 ppm and signal of the methyl group (3H, singlet) at and d 2.31 ppm were also easily detected. Nine signals in the 13C-NMR spectrum of (3) were considered to be accordant with expected structure of the 1-amino-4-(4-chlorobenzylidene)-2-methyl-1H-imidazolin-5(4H)-one. The HR-MS of this product showed the molecular ion peak [M + H]+ = 236.0637, which was agreement with mass calculated for C11H10ClN3O with 235.0512.
1-Amino-4-(4-chlorobenzylidene)-2-methyl-1H-imidazolin-5(4H)-one compound (3) reacted with aromatic aldehydes in absolute ethanol to form azomethine derivatives (4a-h), respectively. Yields, physical properties and IR spectral data of these azomethines were shown in Table 1. The IR spectra of Schiff’s bases (4a-h) obtained from amine (3) showed not only a lack of stretching band at 3227-3424 cm-1 (νNH2) but also an appearance of the absorption band of imine group (C=N) around 1645-1666cm-1. 1H-NMR spectra of (4a-h) compounds show a new signal observed at 9.44-10.07 ppm integrating for the proton of the azomethine group (CH=N) (see Table 2). In the HMBC spectra (see the example of the HMBC correlations of (4g) compound shown in the Figure 2), the singlet around 9.44-10.07 ppm in the 1H-NMR spectra made cross peak with signals of carbon atoms in the benzene ring of benzylidene moiety bonded to nitrogen. The values of chemical shift of the azomethine protons were also similar to values of chemical shift of these protons in the 1H-NMR of 1-arylideneamino-4-arylidene-2-phenyl-1H-imidazolin-5(4H)-one compounds.10 Using the HSQC and HMBC spectra, the other 13C signals of the Schiff’s bases were assigned and summarized in Table 3.
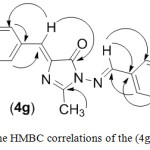 |
Figure 2: The HMBC correlations of the (4g) compound. |
Table 1: Physical and IR spectral data of the Schiff’s bases (4a-h).
|
(Comp.)/X |
M.p. (oC) |
Yield (%) |
IR (n, cm-1) |
[M + H]+ [Calc.] |
||
|
C-H |
C=O |
C=C, C=N |
||||
|
(4a)/ 4-CH3O |
176-178 |
80 |
3060 2930 |
1717 1678 |
1605 1561 |
354.1051 [354.1009] |
|
(4b)/ 4-CH3 |
182-184 |
66 |
2924 2855 |
1705 1651 |
1589 |
360.0867 * [360.0880] |
|
(4c)/ 2-F |
196-197 |
78 |
– |
1717 1651 |
1591 |
342.0824 [342.0809] |
|
(4d)/ 4-F |
184-185 |
74 |
– |
1713 1651 |
1589 |
342.0818 [342.0809] |
|
(4e)/ H |
173-174 |
68 |
2960 |
1713 1651 |
1589 |
324.0928 [324.0904] |
|
(4f)/ 2-NO2 |
175-177 |
77 |
2847 |
1705 1643 |
1582 |
369.0751 [369.0754] |
|
(4g)/ 3-NO2 |
181-182 |
81 |
3079 |
1713 |
1589 |
369.0746 [369.0754] |
|
(4h)/ 4-NO2 |
213-214 |
84 |
2924 |
1705 1643 |
1582 |
369.0783 [369.0754] |
*(4b): [M + Na]+.
Additionally, structure of the Schiff’s bases was further confirmed by HR-MS data. Molecular ion peaks in the mass spectra of the products were conformity with molecular formulas of the desired 1-arylideneamino-4-(4-chlorobenzylidene)-2-methyl-1H-imidazolin-5(4H)-one compounds (see Table 1).
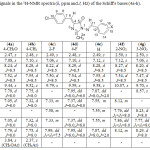 |
Table 2: Signals in the 1H-NMR spectra (δ, ppm and J, Hz) of the Schiff’s bases (4a-h). |
Table 3: Signals in the 13C-NMR spectra (δ, ppm) of the Schiff’s bases (4a-h).
|
X Number |
(4a) 4-CH3O |
(4b) 4-CH3 |
(4c) 2-F |
(4d) 4-F |
(4e) H |
(4f) 2-NO2 |
(4g) 3-NO2 |
(4h) 4-NO2 |
|
2 |
163.3 |
163.6 |
162.8 |
165.3 |
163.6 |
163.3 |
162.6 |
162.9 |
|
2a |
14.8 |
15.2 |
14.5 |
15.2 |
15.2 |
15.1 |
14.5 |
14.7 |
|
4 |
137.2 |
137.3 |
136.8 |
137.2 |
137.2 |
136.9 |
136.6 |
136.7 |
|
5 |
165.6 |
165.9 |
165.6 |
165.9 |
165.9 |
166.0 |
165.4 |
165.5 |
|
6 |
125.8 |
124.9 |
124.9 |
125.0 |
125.0 |
125.5 |
125.0 |
125.2 |
|
7 |
132.4 |
132.7 |
132.2 |
132.6 |
132.6 |
132.5 |
132.1 |
132.3 |
|
8 |
133.3 |
133.7 |
133.3 |
133.7 |
133.7 |
133.8 |
133.3 |
133.5 |
|
9 |
128.5 |
128.9 |
128.4 |
128.9 |
128.9 |
128.9 |
128.3 |
128.4 |
|
10 |
134.7 |
135.0 |
134.8 |
135.0 |
135.1 |
135.2 |
134.8 |
135.0 |
|
11 |
154.7 |
154.6 |
146.6 |
153.1 |
154.3 |
148.8 |
151.1 |
151.0 |
|
12 |
124.5 |
130.9 |
126.3 |
130.3 |
133.6 |
131.9 |
135.2 |
139.5 |
|
13 |
129.2 |
127.7 |
160.1 |
130.1 |
129.0 |
148.5 |
121.3 |
128.5 |
|
14 |
114.3 |
129.6 |
115.3 |
116.1 |
127.7 |
124.7 |
148.1 |
123.7 |
|
15 |
161.8 |
141.6 |
120.9 |
163.0 |
131.5 |
128.6 |
133.2 |
148.7 |
|
16 |
114.3 |
129.6 |
124.6 |
116.1 |
127.7 |
133.8 |
130.1 |
123.7 |
|
17 |
129.2 |
127.7 |
133.0 |
130.1 |
129.0 |
128.0 |
124.9 |
128.5 |
|
others |
55.2 (CH3OAr) |
21.1 (CH3Ar) |
– |
– |
– |
– |
– |
– |
Schiff’s bases (4a-f) were examined for antimicrobial activity against Escherichia coli, Pseudomonas aeruginosa (Gram-negative bacteria); Bacillus subtilis, Staphylococcus aureus (Gram-positive bacteria); Aspergillus niger, Fusarium oxysporum (mold) and Saccharomyces cerevisiae, Candida albicans (yeast). These compounds were subjected to examination of their MIC values according to the reported method12 and the data are shown in Table 4. The two-fold microdilution broth method was used and all of the tested samples demonstrated inhibitory effects in a concentration-dependent manner. However, the results showed that only 4a, 4b and 4h exhibited any significant inhibition against Staphylococcus aureus with MIC values of 100 μg/mL.
Table 4: The minimum inhibitory concentrations (MIC) of the Schiff’s bases against bacteria and fungi.
|
Sample |
MICa (mg/mL) |
|||||||
|
Bacteria Gr (-) |
Bacteria Gr (+) |
Mold |
Yeast |
|||||
|
E.C |
P.A |
B.S |
S.A |
A.N |
F.O |
S.C |
C.A |
|
|
4a |
(-)b |
(-) |
(-) |
100 |
(-) |
(-) |
(-) |
(-) |
|
4b |
(-) |
(-) |
(-) |
100 |
(-) |
(-) |
(-) |
(-) |
|
4c |
(-) |
(-) |
(-) |
(-) |
(-) |
(-) |
(-) |
(-) |
|
4d |
(-) |
(-) |
(-) |
(-) |
(-) |
(-) |
(-) |
(-) |
|
4e |
(-) |
(-) |
(-) |
(-) |
(-) |
(-) |
(-) |
(-) |
|
4f |
(-) |
(-) |
(-) |
(-) |
(-) |
(-) |
(-) |
(-) |
|
4g |
(-) |
(-) |
(-) |
(-) |
(-) |
(-) |
(-) |
(-) |
|
4h |
(-) |
(-) |
(-) |
100 |
(-) |
(-) |
(-) |
(-) |
aE.C – Escherichia coli, P.A – Pseudomonas aeruginosa, B.S – Bacillus subtilis, S.A – Staphylococcus aureus, A.N – Aspergillus niger, F.O – Fusarium oxysporum, S.C – Saccharomyces cerevisiae and C.A – Candida albicans.
bMIC > 100 μg/mL and not determined.
Conclusion
Refluxing mixture of 4-(4-chlorobenzylidene)-2-methyloxazol-5(4H)-one and hydrazine hydrate in different solvents forms different products. In ethanol, the product is 1-amino-4-(4-chlorobenzylidene)-2-methyl-1H-imidazolin-5-one but in pyridine, the product is 3-(4-chlorophenyl)propanohydrazide.
The reaction of 1-amino-4-(4-chlorobenzylidene)-2-methyl-1H-imidazolin-5-one with aromatic aldehydes yielded eight Schiff’s bases, respectively. The structure of the Schiff’s bases was determined by IR, 1H-NMR, 13C-NMR and MS spectral data. Most of the Schiff’s bases exhibited low activity against tested microorganisms including Escherichia coli, Pseudomonas aeruginosa (Gram-negative bacteria); Bacillus subtilis, Staphylococcus aureus (Gram-positive bacteria); Aspergillus niger, Fusarium oxysporum (mold) and Saccharomyces cerevisiae, Candida albicans (yeast).
Conflict of Interests
There is no conflict of interests.
Acknowledgements
This research was not supported by any organizations.
References
- Shalini, K., Sharma, P. K., Kumar, N. Der Chemica Sinica 2010, 1(3), 36-47.
- Bhatnagar A., Sharma P. K., Kumar N. Int. J. Pharm Tech Res. 2011, 3(1), 268-282.
- Gupta, P., Gupta, J. K. Int. J. Modern Chem. 2015, 7(2), 60-80.
- Da Silva, C. M., Da Silva, D. L., Modolo, L. V., Alves, R. B., De Resende, M. A., Martins, C. V. B., De Fátima, A. J. Adv. Res. 2011, 2, 1-8.
- Kajal, A., Bala, S., Kamboj, S., Sharma, N. and Saini, V. J. Catalysts 2013, Article ID 893512. Available from: http://dx.doi.org/10.1155/2013/893512
- Channe G., Ullas, B.J., Chandrashekar, P. G., Suhas, R., Rakesh, K.P., Avinash, P. J. Chem. Applied Biochem. 2015, 2(1), 116-123.
- Azab, M. E., Rizk, S. A., Amr, A. E-G. Molecules 2015, 20, 18201-18218. Available from: https://doi.org/10.3390/molecules201018201
- Nguyen, C. T., Nguyen, D. V., Ta H. T. T., Le H. T. T., Hoang P. T. L., Vuong T. L. A. HCMUE J. Sci. 2014, 58, 20-26.
- Nguyen, C. T., Nguyen D. D., Truong L. N. A., Truong N. T. Q. Vietnam J. Chem. 2015, 53(6e1,2), 315-319.
- Al Abodi, A. J. K., Majed, N., Sahar, A. K., Al-Bayati, R. I. H. Am. J. Org. Chem. 2012, 2(6), 143-150. Available from: DOI: 10.5923/j.ajoc.20120206.04
- Al-Soodani, M. A. A., Ali, A. H. M., Al-Marjani, M. F., Atia, A. K. Int. J. Adv. Res. 2014, 2(3), 399-408.
- McKane, L., Kandel, J. Microbiology: Essentials and Applications. 2nd edition. McGraw-Hill: New York, USA, 1996, 375-406.

This work is licensed under a Creative Commons Attribution 4.0 International License.









