Preparation and Activation of Sarulla Natural Zeolites as an Adsorbent in Purification Process of Crude Palm Oil
Hafni Indriati Nasution1, Moondra Zubir*1 , Jasmidi1, Maharani1, Ahmad Gazali Sofwan2 andJunifa Layla Sihombing1
, Jasmidi1, Maharani1, Ahmad Gazali Sofwan2 andJunifa Layla Sihombing1
1Department of Chemistry, Faculty of Mathematics and Natural Science, State University of Medan, Jl.Willem Iskandar, Ps V, Medan Estate, Medan, North Sumatera 20221, Indonesia.
2Indonesian Oil Palm Research Institute, Medan, North Sumatera, Indonesia.
Corresponding Author E-mail: moondrazubir@unimed.ac.id
DOI : http://dx.doi.org/10.13005/ojc/350228
Article Received on : 26-12-2018
Article Accepted on : 30-03-2019
Article Published : 22 Apr 2019
The effect of Sarulla zeolite in CPO purification was analyzed in zeolite weight and contact time variation on the influence of Free Fatty Acid, Saponification Value, Carotene, Vitamin-E, and Squalene content. This purification process induce increasing of saponification value from 272.41 mg KOH/g to 316.42 mg KOH/g at 20 g of zeolite and contact time for 30 minutes. On the other hand, this purification process reduced of Free Fatty Acid (FFA) content from 2.86% to 1.6% and vitamin E content from 907.79 ppm to 376.54 ppm after were adsorbed for 90 minutes with 30 grams of zeolite used. Fatty acid composition analysis on CPO not significant changes in which is palmitic acid increased or decreased by about 1% under various conditions of zeolite use and contact time on the adsorption process. Sarulla natural zeolite can be used for CPO purification without affect theirs main content.
KEYWORDS:Adsorption; Crude Palm Oil; Natural Zeolite; Purification
Download this article as:| Copy the following to cite this article: Nasution H. I, Zubir M, Jasmidi J, Maharani M, Sofwan A. G, Sihombing J. L. Preparation and Activation of Sarulla Natural Zeolites as an Adsorbent in Purification Process of Crude Palm Oil. Orient J Chem 2019;35(2). |
| Copy the following to cite this URL: Nasution H. I, Zubir M, Jasmidi J, Maharani M, Sofwan A. G, Sihombing J. L. Preparation and Activation of Sarulla Natural Zeolites as an Adsorbent in Purification Process of Crude Palm Oil. Orient J Chem 2019;35(2). Available from: https://bit.ly/2Vkc6st |
Introduction
Indonesia has a variety of natural resources which potential to be developed into various foodstuffs. Oil palm is a plant that grows well in tropical climates with rainfall around 2000 mm/year and temperature range 22-32°C. Currently, 11 million hectares of oil palm plantations in Indonesia have produced crude palm oil with a production capacity of 31.10 million tons per year and it is the largest of palm oil production in the world.1 CPO (Crude palm oil) is a crude oil obtained by extraction of palm flesh and usually still contains dissolved impurities and not dissolved in oil. This impurities known as gum or resin contain of phosphatides, proteins, hydrocarbons, carbohydrates, water, resins, free fatty acids (FFA), tocopherols, pigments and other compounds. CPO is also known rich with natural dyes as α-carotene, β-carotene, xanthophyll, chlorophyll and anthocyanin. These dyes cause oil colour become yellow, yellow-brownish, greenish and reddish.2
Excessive dye content on CPO will degrade the quality and affect the physical appearance, taste, odor and oil storage time, so it must be removed by physical or chemical separation process. The process of purification is one of strategic to reduce or eliminate dyestuffs in crude oil (CPO), whether dissolved or dispersed. The color of the crude oil can be derived from the color of the congenital oils or the colors arise during the processing. One of the alternative solutions to reducing carotene or dyestuff content in CPO is purification by adsorption method. This process would absorb carotene in CPO by using adsorbent material as clay and activated carbon.3
Beside activated carbon,4 zeolite also can use as an absorbent but not many research have used zeolite as an adsorbent for CPO purification.5 This ability due to the presence of SiO2 and AlO2 on the surface of the adsorbent to adsorb the β carotene in palm oil.6 Indonesia has a large reserve of natural zeolite that spread around 50 locations, especially around volcano area like in East Java, Central Java, West Java, Borneo, Nusa southeast, Maluku to Sumatra which potentially have deposits of 16.6 million tons.1
In this research, natural zeolite from Sarulla village, Pahae Jae district in North Sumatera was used as an adsorbent in CPO purification to improve the quality of palm oil. This purification was performed by mixing CPO with variation of zeolite amount and variation of adsorption time. CPO feed and after purification were determined of Free Fatty Acid (FFA) content, Saponification Value (SV), carotene content, vitamin E content7 and squalene content.8 Fatty acid composition was observed to confirm this purification process will not change the quality of palm oil. The utilization of natural zeolite as an adsorbent for palm oil was expected to promote this natural zeolite on produce more pure and high quality of CPO.
Material and Methods
CPO feed in this experiment was taken from Adolina, one of palm oil Company in North Sumatera. Sarulla zeolite, n-hexane, HCl 0.5 N, HCl 1N, Oxalic Acid (H2C2O4), Phenolphthalein Indicator, Sodium Tetraborat (Na2B4O7.10H2O), Ethyl Alcohol, KOH 0,01 N, KOH Alcoholic 0.5 N, Vitamin C 0,1%, KOH 50%, Ethanol 40%, NaOH Methanolic 0,5 N, BF3, Methanol, NaCl saturated and aquadest were used in this experiment.
Preparation of Sarulla Zeolite
Natural zeolite was collected from Sarulla, Tapanuli Utara area and grilled with mortar and pestle, sieved with 100 mesh size to obtain zeolite grain size less than 100 mesh. Then zeolite was washed with aquadest and dried in oven at 110 oC for 3 hours. Prepared zeolite were kept in desiccators and measured of initial crystal structure by XRD.
Activation of Sarulla Zeolite
100 g prepared zeolite was mixed with 200 mL of HCl 1 N and stirred at 150 rpm for 3 hours. The zeolite was filtered by using filter paper whatman No.42 and rinsed with aquadest until neutral pH and dried it at 110°C for 3 hours. This process was continued with the calcination process at 300°C for 2 hours to obtain activated natural zeolite. This activated zeolite was characterized with XRD analysis.
Purification of CPO by Sarulla Zeolite
Purification of CPO by using activated Sarulla zeolite was measured by weighing each 100 g of CPO, and put into a 250 mL beaker glass. 10 g, 20 g, and 30 g activated Sarulla zeolite was added and heated at 90 °C by stirring for 30, 60, and 90 minutes. CPO after adsorption was filtered and characterized as below parameter.
Characterization
Change of Zeolite structure after activation was measured by X-Ray Diffractometer, XRD Shimadzu 6100. Carotene content was measured by UV-Visible Spectrophotometer (UV-Vis) and squalene content was determined by Gas Chromatography Mass Spectrometry (GC-MS). High Performance Liquid Chromatography (HPLC) was used to measure Vitamin E content and Gas Chromatography (GC) to determine composition change of fatty acid after purification.
Results and Discussion
Activation by using HCl aims to dissolve acid-soluble impurities such as alkaline metal oxide compounds. Activation with acid causes de-cationation to increase zeolite surface area with reduce the impurities which cover the pores of zeolite. Using low concentrations of HCl in the zeolite activation process to avoid the structural damage. Zeolite was immersed in HCl, then filtered with filter paper and washed with aquadest until neutral pH removed Cl- in the activated zeolite cavity. To confirm it was investigated with addition of AgNO3. No sedimentation after the addition of AgNO3 indicates zeolite is completely free of Cl–. Then it was calcined to evaporate the trapped water in zeolite pores, burning the organic compounds contained in the zeolite and it induce the zeolite surface area increases, and to obtain a good form of crystallinity. Figure 1 shows the XRD pattern of Sarulla natural zeolite from the preparation process and the activation process.
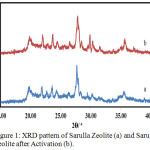 |
Figure 1: XRD pattern of Sarulla Zeolite (a) and Sarulla Zeolite after Activation (b). |
Prepared Sarulla Zeolite shows high intensity around 27.70°, 23.57°, 19.81°, 22.67°, and 35.50°. On the other hand, activated Sarulla zeolite was observed a high intensity at 27,83°, 23,69°, 29,86°, 19,84°, and 35,66°. Some peaks of decreased intensity indicates loss of impurities which caused by activation process. XRD pattern also confirm that activation process of Sarulla zeolite is not destructive and does not alter the zeolite structure characterized by no significant change in XRD peaks. The activation process was succeed to remove impurities contained in the Sarulla natural zeolite. According to EXPO 2014 analysis,9 the same crystalline system of the Sarulla natural zeolites after preparation and after activation form a triclinic crystal system but the volume of crystals system after activation was greater than after preparation. Its supported by XRD data that the activation of Sarulla natural zeolite was successfully remove the impurities.
Characteristics of the palm oil quality was influenced by its free fatty acid content (Figure 2). For calculations, the FFA content of palm oil is considered as Palmitic Acid (molecular weight 256) and high levels of FFA indicate poor quality of oil. FFA was measured by volumetric titration method where analysis of the amount of free fatty acid of a sample equivalent to the amount of base (KOH) added in the titration, indicated by the color change.10 Free Fatty Acid (FFA) on CPO before the purification process is 2.86%. After purification with Sarulla zeolite, the optimum FFA value was decreased to 1.60% at 30 g Sarulla zeolite and 90 minutes contact time . It indicate the adsorbent weight and contact time in CPO refining process induce to the decrease of FFA content. By increasing of Sarulla zeolite weight and contact time will effective to purify of CPO which significant influence on the quality of CPO. Reducing of FFA value is significance around 44.1%, higher than purification process of Zeolite from Klaten which reduced of FFA value as 17.5 %.15
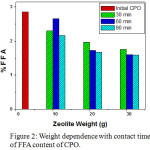 |
Figure 2: Weight dependence with contact time of FFA content of CPO. |
Saponification Value is the amount of (mg) KOH required to soap one gram oil or fat, and alcohol presence in KOH to dissolve the fatty acids from the hydrolysis and facilitate the reaction with the base to form soap.11 The greater of saponification value induce the lower fatty acids and better oil quality, otherwise if the saponification value is low, it increase the fatty acid and quality of CPO was decreased. The saponification value of initial CPO is 272.41 mg KOH/g, but after added of adsorbent and longer contact time observed the increasing of saponification value. The smallest Saponification Value after the addition of adsorbent was obtained at 10 g zeolite as 283.91 mg KOH/g with contact time 60 minutes and the highest saponification value at zeolite mass 20 g and 30 increase the saponification value to produce more quality of oil palm.
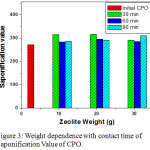 |
Figure 3: Weight dependence with contact time of Saponification Value of CPO. |
The main carotenoids of palm oil are alpha-carotene and beta-carotene; together contain 80 % of the total carotenoids in palm oil with 36.4 % alpha-carotene and 54.4 % beta-carotene.9 CPO has a high carotene content around 500-700 ppm. Beside as a color given, carotene also acts as an antioxidant in the body and serves as a precursor of vitamin A especially for β-carotene formation.12 Codex Alimentarius Commission (2003) used as a reference in international trade specifies that the requirement of carotene content ranges of CPO around 500-2000 ppm. Figure 4 shows the highest carotene content was possessed by the initial CPO sample with a carotene content is 460 ppm and the lowest is a variation of zeolite mass of 30 grams and contact time of 60 minutes and 20 g of zeolite mass with contact time of 90 minutes as 5 ppm carotene content. The longer stirring time on the zeolite and increase of zeolite amount to purify the CPO will reduce carotene content. The differences of carotene content and drastic decrease after purification were caused by several factors, as palm oil varieties and maturity level of palm fruit. It suggest the purified CPO will lose its carotene content, indicates the purified CPO was not recommended for food and beverage consumption due to very low carotene content, but can be used in other industries such as cosmetics and medicine.7,13
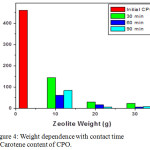 |
Figure 4: Weight dependence with contact time to Carotene content of CPO.Click here to view figure |
Purified CPO was determined of reduction vitamin E content as 376.54 ppm in the 30 gram zeolite and 90 minute contact time compared to initial CPO is 907.79 ppm (Figure 5). It suggest by increasing zeolites weight will effect more vitamin E adsorbed by zeolites and produce lower levels of vitamin E. The highest vitamin E content of purified CPO was measured in 20 g Sarulla zeolite and a contact time of 30 minutes is 808.01 ppm. The content of d-tocotrienol and a-tocopherol levels in CPO induce the high vitamin E produced, and g-tocotrienol and a-tocotrienol level of purified CPO is lower than the initial CPO.14
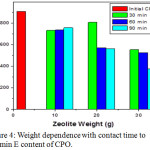 |
Figure 5: Weight dependence with contact time to Vitamin E content of CPO. |
The purified crude palm oil had elevated levels of squalene content as 247.51 ppm compared than the initial sample is 162.99 ppm. After purification with Sarulla natural zeolite the level of squalene increase, but on the addition of 20 gram zeolite with stirring time of 90 min squalene level decreased to 244.68 ppm, although squalene level decreased in the variation, purification CPO remains better because the squalene content is higher than intial CPO. In this study the analysis results of squalene levels increased to 65% where 60 minutes contact time and used 20 gram zeolite. Its suppose that purified CPO with high squalene content is more suitable to use as a cosmetic ingredient.8
The analysis of fatty acid composition aims to determine the percentage of saturated fatty acid (C: 12 – C: 18) and unsaturated (C: 18.1 – C: 18.3) contained in Crude Palm Oil. Initial CPO was determined of oleic acid content (C18: 1) about 38% while CPO adsorption use 30 g and 90 minutes contact time, oleic acid decreased to 37% and 30 grams with 60 minutes contact time, oleic acid increased to 39%. For palmitic acid content at initial CPO only 46% but purified CPO with 20 gram and 60 minute contact time decreased to 44% as palm oil with lowest palmitate content, while on purified CPO with variation 20 gram 30 minutes, palmitic acid content increased to 47%.
Table 1: Fatty Acid composition of purified CPO.
| Fatty Acid Content in CPO | Measurement Parameter | |||||||||
| zeolite weight (g), contact time (min) | ||||||||||
| Initial | 10,30 | 10,60 | 10,90 | 20,30 | 20,60 | 20,90 | 30,30 | 30,60 | 30,90 | |
| Laurat Acid | 0,16 | 0,16 | 0,17 | 0,16 | 0,34 | 0,15 | 0,15 | 0,18 | 0,19 | 0,66 |
| Miristat Acid | 0,80 | 0,77 | 0,80 | 0,81 | 0,89 | 0,71 | 0,76 | 0,80 | 0,74 | 1,02 |
| Palmitat Acid | 46,27 | 45,24 | 46,21 | 45,65 | 47,54 | 44,30 | 45,83 | 45,24 | 44,34 | 46,20 |
| Palmitoleat Acid | 0,11 | 0,11 | 0,11 | 0,11 | 0,12 | 0,12 | 0,11 | 0,12 | 0,11 | 0,23 |
| Stearat Acid | 3,73 | 3,83 | 3,81 | 3,88 | 3,83 | 3,66 | 3,87 | 3,81 | 3,99 | 3,90 |
| Oleat Acid | 38,09 | 38,84 | 37,99 | 38,40 | 38,00 | 39,81 | 38,30 | 38,47 | 39,82 | 37,34 |
| Linoleat Acid | 10,28 | 10,48 | 10,33 | 10,42 | 10,22 | 10,71 | 10,39 | 10,80 | 10,21 | 10,25 |
| Linolenat Acid | 0,17 | 0,18 | 0,18 | 0,19 | 0,18 | 0,19 | 0,18 | 0,19 | 0,19 | 0,19 |
| Arachidat Acid | 0,28 | 0,29 | 0,29 | 0,29 | 0,23 | 0,27 | 0,29 | 0,29 | 0,31 | 0,29 |
| Eicosenoat Acid | 0,08 | 0,09 | 0,08 | 0,09 | 0,09 | 0,08 | 0,09 | 0,08 | 0,10 | 0,09 |
Conclusions
Purification of CPO by using Sarulla natural zeolite successful in decreasing free fatty acid content from 2,86% to 1.6%, increasing saponification value from 272.41 to 316,42 mg KOH / g and also increase of squalene from 162.99% to 247,5. However, even this purification induce decrease of carotene levels to 5 ppm and vitamin-E content to 376.54 ppm, but CPO purified by Sarulla natural zeolite is suitable for the manufacture of drugs and cosmetics. This purification process not significant effect to composition of fatty acids which palmitic acid has a slight increase of 1% and a decrease of 1% in some experimental variation conditions. It could be concluded, Sarulla natural zeolite is suitable for CPO purification.
Conflict of Interest
There is no conflicts of interest.
References
- Petrenko; Julia P; Stephanie S., International Council on Clean Transportation, 2016.
- D.J. Osborne; J.Henderson. Journal endevour, 2000, 24, 63-68.
- Simone M.S; Roberta C; Roland V; Christian S; WimDe G; Antonio J.A.M. Journal of Food Engineering, 2013, 341-349.
- Agus K; Moondra Z; Jasmidi; Albinus S. Asian Journal of Chemistry, 2018, 30, 5, 944-946.
- Asalil M; Gede W; Mukhammad F.N.; Miftakhul F. Indonesian Journal of Chemistry. 2014, 14, 2, 138-142.
- Maria U; Sri R; Pudji H; Purnama D. AIP Conference Proceedings. 2016, 1755, 130016.
- Chu B.S.; Quek S.Y.; Baharin B.S. Food Chemistry, 2003, 80, 3, 295-302.
- Derek M.; Armelle P.; Lance K.; Loren P. Cosmetics & Toiletries Magazine, 2014, 129(6).
- Altomare, A.; Cuocci, C.; Giacovazzo, C.; Moliterni, A.; Rizzi, R.; Corriero, N.; Falcicchio. J. Appl. Cryst., 2013, 46, 1231-1235.
- Mahesar S. A.;. Sherazi S. T. H ; Khaskheli A.R,; Aftab A.K.; Kandhro; Sirajuddin. Analitycal method, 2014, 6, 14-18.
- Mustapha Abdulrahman. IOSR Journal of Applied Chemistry (IOSR-JAC) : 2278-5736. 2016, 9, Issue 1 Ver. I.
- Latip R. A.; Baharin B. S.; Che Man Y. B.; Abdul Rahman R.. Journal of the American Oil Chemists’ Society, 2001, 78 (1), 83-87.
- Ooi C. K.; Choo Y. M.; Yap S. C.; Basiron Y.; Ong A. S. H.. Journal of the American Oil Chemists’ Society, 1994, 71 (4), 1994, 423-426.
- Ab Gapor,M.T. Palm Oil Development , 1990, 12, 25-27.
- Monika L.; Putranti T.A.; Wirawan S.K.; I Made Bendiyasa, IOP Conf. Series: Materials Science and Engineering, 2018, 299-304.

This work is licensed under a Creative Commons Attribution 4.0 International License.









