Physico-Chemical Investigation of Aqueous Polymer Solutions and Their Application as Soft Medicinal Forms
Nagima Dzhakipbekova , Botagoz Torlanova, Erzhan Dzhakipbekov, Laura Aykozova, Asel Bekmurzaeva, Esbol Ordabek, Madina Fatkullina and Beysen Shyngisbaev
, Botagoz Torlanova, Erzhan Dzhakipbekov, Laura Aykozova, Asel Bekmurzaeva, Esbol Ordabek, Madina Fatkullina and Beysen Shyngisbaev
M. Auezov South Kazakhstan state university, Shymkent, Republic of Kazakhstan
Corresponding Author E-mail: isa.aziza@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/350218
Article Received on : 20-06-2018
Article Accepted on : 01-04-2019
Article Published : 27 Apr 2019
The growing demand for water-soluble polymers of the acrylic series causes the researchers' interest in the synthesis of these compounds, which ultimately allows solving the tasks of the productivity increase, finding ways to improve the quality of final products, developing new highly efficient technological processes for the synthesis of these substances and their application as soft medicinal forms.
KEYWORDS:Antibiotic; Density; Polyacrylamide; Polyelectrolytes; Viscosity
Download this article as:| Copy the following to cite this article: Dzhakipbekova N, Torlanova B, Dzhakipbekov E, Aykozova L, Bekmurzaeva A, Ordabek E, Fatkullina M, Shyngisbaev B. Physico-Chemical Investigation of Aqueous Polymer Solutions and Their Application as Soft Medicinal Forms. Orient J Chem 2019;35(2). |
| Copy the following to cite this URL: Dzhakipbekova N, Torlanova B, Dzhakipbekov E, Aykozova L, Bekmurzaeva A, Ordabek E, Fatkullina M, Shyngisbaev B. Physico-Chemical Investigation of Aqueous Polymer Solutions and Their Application as Soft Medicinal Forms. Orient J Chem 2019;35(2). Available from: https://bit.ly/2IVFHlz |
Introduction
Copolymers of acrylic acid, which are well mixed with water forming viscously fluid mass were synthesized on the basis of the Department of Physical and Colloid Chemistry in M.Auezov SKSU. Their properties and consistency are of interest for research in terms of the possibility of their use as auxiliary substances in pharmaceutical technology in the preparation of external dosage forms.
The drugs were toxicologically tested. Based on the conducted toxicological studies and analysis of the data obtained, it was concluded that the polymers possess little functional cumulation. They do not show skin-resorptive and allergic action.
IPAA-PV, IPAA-MEA
The viscosity (ŋ rel) of 0.2% solution is 4.12 – 5.64. With time, viscosity increases. 24 hours after the synthesis, it was 5.64; after the 30 days expiration ŋ = 4.12, that means it increased to 36 times.1 This property can be used in the preparation of films.
Granular Polyacrylamide (GPAA)
GPAA is an irregularly shaped yellowish or slightly brown granules, the polymer has a polymer mass fraction of 50-56%, moisture content 10-14%, ammonium sulfate 30-40%. pH of 0.1% aqueous solution is 7-8. The kinematic viscosity of 0.1% aqueous solution is not less than (1.7 ± 2) 10 -6.2,3
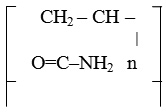
Materials and Method
The optical density was recorded on a spectrophotometer SF-26.
The turbidity was determined on a FEK-56 calorimeter at α = 434 Nm. solutions of the corresponding polymer fractions were used as comparison solutions.
The effect of the PAA, PV and MEA based polymers mixtures ratio in this case is manifested in the fact that when the content of PE based on PAA the ratio of PV- MEA increases, the optical density of sludge also increases, and with decreasing of their content, the optical density of filtrates practically does not change.
Table 1: Polymers optical density of investigation results.
| Polymer | % solution | additive | density |
| IPAA-IEA | 2.5 | МЭА IEA | 1-0.11592-0.11613-0.11694-0.1178
0.1181 Dist.water -0.1215 |
| IPAA-IEA | 5 | МЭА IEA | 1-0.11642-0.11923-0.11974-0.1203
5-0.1207 Dist.water -0.1235 |
| MPAA-PV | 2.5 | Hydrogen peroxide | 1-0.11132-0.11553-0.11614-0.1163
5-0.1165 Dist.water -0.1189 |
| MPAA-PV | 5 | Hydrogen peroxide | 1-0.11202-0.11473-0.11524-0.1155
5-0.1161 Dist.water-0.1174 |
| MPAA-PV | 2.5 | Hydrogen peroxide | 1-0.11612-0.11583-0.11714-0.1172
5-0.1190 Dist.water -0.1228 |
| MPAA-PV | 5 | Hydrogen peroxide | 1-0.10132-0.11673-0.11904-0.1178
5-0,1166 Dist.water -0.1194 |
| IPAA-IEA | 2.5 | IEA | 1-0.12012-0.12113-0.12244-0.1226
5-0.1234 Dist.water -0.1256 |
| IPAA-IEA | 5 | IEA | 1-0.11592-0.11833-0.12084-0.1212
5-0.1213 Dist.water-0.1240 |
| MPAA-TEA | 2.5 | TEA (1 ml) | 1-0.13752-0.11783-0.14724-0.1572
5-0.1180 Dist.water -0.1580 |
| MPAA-TEA | 5 | TEA (1 ml) | 1-0.11702-0.13753-0.14734-0.1374
5-0.1473 Dist.water -0.1480 |
| MPAA-TEA | 2.5 | TEA (0.5 ml) | 1-0.08822-0.08823-0.09814-0.0981
5-0.179 Dist.water -0.0990 |
| MPAA-TEA | 5 | TEA (0.5 ml) | 1-0.09812-0.09803-0.10804-0.1079
5-0.1277 Dist.water -0.1280 |
The increase in optical density of PAA and PV, MEA polyelectrolytes can be explained by conformational transformations of macromolecules. For samples of the MPAA-TEA, the optical density value in all the studied concentration intervals varies insignificantly (Table 1), which indicates the homogeneity of the solutions and their thermodynamic stability.
Study of Viscous Properties of GRP by Viscometry
The viscosity of polyelectrolytes solutions was measured in a Ubbelohde viscometer, with a suspended level. Re-precipitated and thoroughly dried polymers were used for viscosimetric studies.
The viscometer was placed in a thermostat, the temperature was maintained with an accuracy of ± 0.01°C.
The concentration of the solution after dilution was calculated from formula
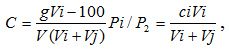
where g is the polymer weight, g;
V is the volume of the volumetric flask, ml;
Vi is the volume of the solution filled in viscosimeter, ml;
VJ – volume of the added solvent, ml;
Pi/PJ is the ratio of the solvent densities.
The calculation of the relative (ηrel), inherent (ηinh) and reduced (ηred) viscosities was carried out according to the following formulas:
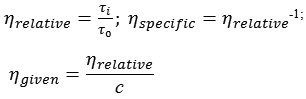
The intrinsic viscosity (η) was found from the graphical dependence of ηred = t (C) by extrapolating the lines to the zero polymer concentration. (3).
Studies of hydrodynamic properties of hydrolyzed mixtures of MPAA-PV in a wide range of concentrations (0.001-1), pH, temperature (Figure 1.2), etc., show that polymer mixture solutions obey the general patterns characteristic of polymer solutions containing ionizing functional groups. Dependences of the specific and reduced viscosity (η red /c) on the concentration of solutions mixture have an anomalous shape of the curves, which is typical for high molecular weight poly electrolytes. This is due to the influence of ionizable hydrophilic functional groups on the hydrodynamic volume of macromolecular coils.
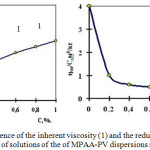 |
Figure 1: Dependence of the inherent viscosity (1) and the reduced viscosity (2) on the concentration of solutions of the of MPAA-PV dispersions mixture. |
The deviation of the reduced viscosity from the linear dependence in the region of dilute solutions of MPAA-PV is due to the fact that the dissociation of ionogenic groups and the electrostatic repulsion of the likely charged functional groups in the polymer chain are intensified with dilution, which leads to straightening of the macromolecular coil and, consequently, to an increase in the hydrodynamic volume and viscous resistance of the system.
The changes in viscosity at a constant ionic strength produced by electrolyte additives, which suppresses the ionization of functional groups and, accordingly, changes the conformational state of macromolecular coils, provide a linear dependence of the reduced viscosity on concentration.
The hydrodynamic properties of solutions of poly electrolytes, especially poly ampholytes, are significantly affected by the pH of the medium and the degree of ionization (Figure 2). The shape of the ηinh/c influencial curves for polyelectrolytes MPAA-PV solutions and pH produced by acid or alkali additives shows the dependence of the hydrodynamic volume of macromolecules on the ionization of functional groups. The maximum of η inh / c-pH curves corresponds to pH = 7.5 – 7.8 and coincides with the data for MPAA-PV, etc. In this pH range the macromolecule assumes the most unfolded conformation due to the ionization of the functional groups and the optimal value of the ionic strength. All preparations in this pH range exhibit the properties of poly anions.4-6
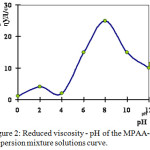 |
Figure 2: Reduced viscosity – pH of the MPAA-PV dispersion mixture solutions curve. |
In all GRP MPAA-PV, the viscosity decreases with increasing ionic strength in the alkaline range pH> 10 (behind the maximum point), which is associated with an excess of low-molecular-weight electrolyte ions and an increase in the ionic strength of the solution.
With an increase in the acidity of the medium, the dissociation of the acidic groups is suppressed, the intramolecular interaction is enhanced by the formation of hydrogen bonds between the amide and carboxylate groups, the macromolecular coils become denser, the interaction with solvents decreases, which leads to a drop in viscosity at pH = 3. A similar pattern is observed in the samples of GRP MPAA-PV obtained in an aqueous solution of sodium hydroxide and in various alcohols (normal isobutyl and normal isopropyl alcohol) in the acidic medium at pH 3.5; then a deposit precipitates in the form of flakes, and at pH = 2.5-3.0 the dissolution of the deposit begins.
At pH = 1.97-3.0, viscosity increases, and at a pH less than 1.97, the viscosity decreases.
A further increase in the amount of hydrochloric acid results in the folding of the polymer macromolecules and formation of a precipitate in the form of flakes, which then dissolves. At low pH values (isoelectric point) macromolecules are usually found in densely folded coils or in the form of dense fibrils due to internal and intermolecular hydrogen bonds, which can be easily destroyed by ionization and the macromolecule is straightened, which leads to an increase in the size of the globules and, accordingly, the viscosity of the GRP solutions increases.
Microbiological Method of Direct Diffusion Into Agar
To establish the compatibility of IPAA with levomycetin sodium succinate and gentamycin sulfate antibiotics it is necessary to detect the antimicrobial activity of antibiotics, which is determined by diffusion into agar.
The method for carrying out this research is described in detail in the USSR pharmaceutical regulations XI ed ., v.II. Due to the fact that it does not take into account some of the features of our experiment (for example, the determination of antimicrobial activity in ointments), we modified the method in order to be able to apply it in our work.
The method of diffusion into agar is based on comparing the degree of inhibition of the test microbe growth zone with certain concentrations of the antibiotic in the test material with inhibition of its growth zone by the known concentrations of the antibiotic standard. The suppression of the test microbe growth is due to the antibiotic diffusion from the test material into a dense nutrient medium.
To obtain reproducible results, strict standardization of experiments is necessary. The rate of solutions diffusion in agar depends on the chemical nature of the antibiotic, the composition, pH of the agar medium, the buffer in which the working solutions of the standard and the test material are prepared, the temperature and incubation time.7-9 Therefore, when determining the concentration of antibiotics and maintenance of their antibacterial activity in test samples we should select the conditions for culturing the test culture, the optimal composition and pH of medium, buffer solutions, ensuring the maximum diffusion of the solutions of antibiotic into the medium and the sharpness of the zones outlines. The determination is carried out according to the scheme common to all antibiotics.
Spectrophotometric quantitative determination of levomycetin sodium succinate in antibacterial ointments.
This highly sensitive method of quantitative determination of the drug in solution is described in Pharmaceutical standard-42-737-78.
The optical density of the 0.002% preparation solution was measured on the SF at a wavelength of 275 nm compared to water in a sample-holder with a layer thickness of 1 cm. The same measurement was carried out with a 0.002% solution of a standard sample at a wavelength of 278 nm.
The percentage (X) of levomycetin in the preparation is calculated by the formula:
![]()
Where
D- is the optical density of the test solution
D0- is the optical density of a solution of a standard sample
C- is the concentration of the test solution
C0- is the concentration of the solution of the standard sample.
The content of levomycetin in the preparation should be at least 65%. Each flask should contain at least 90% and not more than 110% of the amount indicated on the label.10
Note: The standard sample is levomycetin (Pharmaceutical Standard for Levomycetin p. 371).
Fractional Sterilization (Tundalization)
The objects to be sterilized are heated at 30°C for 1 hour every 24 hours. In the intervals between heating the object is kept in a thermostat at a temperature favorable for the spore germination (25-37°C).
Three cycles are enough for all the spores contained in the object to germinate and die with subsequent heating.
The Method of Equilibrium Dialysis According to Kruvchinsky
The dialysis device proposed by Kruvchinsky consists of a beaker that places 50 ml of the model medium, a dialysis tube with an internal diameter of 32 mm and a height of 160 mm. Put 1.0 g of the test sample of the ointment in the lower end of the tube. Put the tube into a glass with a dialysis medium to a depth of 2 mm. A thermometer for temperature control and a pipette for sampling are put into the glass. The glass is placed in a water bath. Samples have volume of 2 ml.
Results and Discussion
Development of the Ointment Base Optimal Composition
When developing the ointment base optimal composition, 1.0 g of MPAA polymer was dissolved in different volumes of distilled water: 1 ml, 2 ml, 3 ml, 4 ml, 5 ml and 6 ml.
It was found out that to obtain an ointment base with the necessary consistency, the optimal ratio of MPAA polymer and distilled water was 1: 5.
The pre-ground and screened MPAA polymer powder was weighed on a BP-1 scale in an amount of 1.0 g, transferred into a mortar and triturated with 5 ml of distilled water, added in parts. As a result of a ten-minute stirring, a thick, sticky, gel-like, transparent mass without odor, yellowish-cream color was obtained. The base is easily applied to the skin, when drying it forms a thin protective film on it, easily removable with a cotton swab dipped in water. The base does not spoil underwear and clothes, does not have irritating effect and does not tighten the skin when it dries.
Thus, the technology of this synthetic hydrophilic base preparation is extremely simple, does not require special methods of preparation and compliance with a lot of conditions. It is convenient and quick to use.
Determination of the MPAA new Synthetic Hydrophilic Base of Resistance to Microbial Contamination
Many known ointment bases are easily exposed to microbial contamination (hydrogenated fats, fatty and vegetable oils, gelatin gels and others). The effect of the antibacterial ointments we are researching is aimed at the destruction of pathogenic microorganisms and products of their vital activity, which are the cause of infectious and inflammatory processes in the wound.
Therefore, one of the most important requirements expressed to ointment bases, is resistance to microbial contamination, because the latter can significantly reduce the concentration of antibiotics in ointments, thereby reducing the therapeutic effect of the preparations, and can be the cause of secondary infection of the wound or burn surface.
In connection with the above, it seems necessary to conduct research in this direction, using a microbiological agar method.
For this purpose, 1 g of MPAA base was added to two tubes containing 4 ml of molten and cooled to 50°C nutrient medium. The content of the tube was quickly and thoroughly mixed and transferred to a Petri dish containing 20 ml of a solidified aerated media.
Spread the top layer of agar evenly with a quick rocking of the Petri dish. After solidifying of the medium, the plates were incubated in a thermostat for 5 days at 35°C.
During this period of time growth of the bacteria was not detected. Consequently, there are less than 10 bacteria in 1 g of polymer base. The permissible limit of microorganisms’ content in medicinal forms of local use is the presence of not more than 100 microorganisms per gram of the drug.
Conclusion
The macromolecules of MPAA-PV polyelectrolytes at pH 3.5-4.0 are in a folded state corresponding to the isoelectric state of the macromolecule. When the pH changes, the macromolecules change their conformation.
At pH 4.0-4.5, the macromolecule straightens, and this contributes to an increase in the viscosity of the solution, the maximum value of the viscosity in all samples is observed at pH = 7.0-8.0.
Compatibility of IPAA with antibiotics of levomycetin sodium succinate and gentamycin sulfate has been established as well as antimicrobial activity of antibiotics.
References
- Tager A.A. Physics and chemistry of polymers. Moscow.: Goschimizdat, 1992, p.23-26.
- Fuoss R, Strauss U.J. Polymer. Sci., 1998, 3.246.
- Febbereitor K.J. Temperature dependence of surface tension // Coll.and Polym. Sci., 1978. V 256., №5, p.490-493.
- Belikov V.G. Pharmaceutical chemistry: A textbook for pharmaceutical institutes and pharmaceutical faculties of medical institutions. -M.: Higher School, 1985, 768.
- Bilibin A.F. Clinical chemotherapy. – Moscow: Medicine, 1983, 511 p.
- Dmitrieva VS, Semenov S.М. Microbiological analysis of antibiotic activity. -M .: Medicine, 1984, 284.
- Kashkin P.N, Bezborodov A.M, Elinov N.T. Antibiotics. -M .: Medicine, 1970.
- Pyatkin K.D. Microbiology with virology and immunology. – Moscow: Medicine, 1981, 347.
- Tentsova A.I, Azhgikhin I.O. Dosage forms and therapeutic activity of drugs. – Moscow: Medicine, 1974, 336.
- Kondratieva T.S., Ivanova L.A, Zelikson Yu.I. Technology of medicinal forms. t.1. – M .: Medicine, 1991.

This work is licensed under a Creative Commons Attribution 4.0 International License.









