Molecular Interaction of Aqueous Solution of 1-Propanol and 2-Propanol
Sandhya Sharma* , N. D. Kandpal and R. Joshi
, N. D. Kandpal and R. Joshi
Department of Chemistry, Kumaun University S.S.J. Campus. Almora, 263601 Uttarakhand India.
Corresponding Author E-mail: bhardwajsandhya8@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350256
Article Received on : 01-12-2018
Article Accepted on : 31-03-2019
Article Published : 18 Apr 2019
Viscosities (ƞ) of solutions of 1- propanol and 2-propanol have been determined in aqueous systems of varying composition (0.16-3.64 mol dm-3) at 298 K. The ƞ/ƞ0 data have been analyzed in the light of reduced Jone-Dole equation ƞ/ƞ0 = BC+1. The results in the regards to solute-solvent interaction in aqueous solutions of both the alcohols have been discussed in the terms of the value B. The result of study reveals that both the alcohols behave as the structure makers in the water. The result shows that solute-solvent type interaction of same magnitude exists in water-alcohol system.
KEYWORDS:Propanol; Reduced Jone-Dole Equation; Solute-Solvent Interaction; Viscosity B-Coefficient
Download this article as:| Copy the following to cite this article: Sharma S, Kandpal N. D, Joshi R. Molecular Interaction of Aqueous Solution of 1-Propanol and 2-Propanol. Orient J Chem 2019;35(2). |
| Copy the following to cite this URL: Sharma S, Kandpal N. D, Joshi R. Molecular Interaction of Aqueous Solution of 1-Propanol and 2-Propanol. Orient J Chem 2019;35(2). Available from: https://bit.ly/2IsrZHq |
Introduction
Viscosities in aqueous solutions of different concentration have been observed and data has been utilized to determine the presence of molecular interaction like ion-ion, solute-solute and solute-solvent present in the system.1-3 The viscosity parameters A and B explore solute-solute and solute-solvent interaction in different system. The aqueous methanol solution has been used as solvent for different solutes in conductomertic or viscometric studies.4-8 The Jone-Dole equation has been utilized to obtain the value of A and B in case of methanol–water system.9 The present work has been undertaken in an attempt in the extension of the viscometric studies of alcohols 1-propanol and 2-propanol in order to investigate the nature of molecular interactions in aqueous solutions considering ion-ion interaction negligible.
Materials and Methods
The alcohols used were of GR grade (E. Merck). Doubly distilled water was used to prepare solutions of alcohols of required concentration. The viscosity measurements were made in calibrated suspended-level viscometers (Infusil India Pvt. Ltd., BG43500 size2 and BG43499 size1). The viscometer was placed in a thermostated water bath (Tanco) having accuracy of ± 0.1 K for maintaining constant temperature. The solutions of alcohols of known concentration were taken in the viscometer and the flow time of the solution was measured with the help of a stopwatch (Racer)10. Each measurement is repeated thrice and an average time of flow was used to calculate the viscosity. The densities of solutions were measured using 15 ml double arm pyknometer having accuracy ±0.00001 g/ml and a single pan electronic balance (citizen).
Results and Discussion
The values of ƞ/ƞ0 at different concentration of alcohols were observed and given in the Table 1 and Table 2.
Table 1: Variation of ƞ/ƞo with concentration of 1-propanol at temperature 298K.
|
S. No. |
Concentration (mol dm-3) |
ƞ/ƞ0 |
√C |
(ƞ/ƞ0-1)/√C |
|
1 |
0.16 |
1.05 |
0.40 |
0.12 |
|
2 |
0.32 |
1.11 |
0.56 |
0.19 |
|
3 |
0.48 |
1.20 |
0.69 |
0.28 |
|
4 |
0.64 |
1.23 |
0.80 |
0.28 |
|
5 |
0.80 |
1.28 |
0.89 |
0.31 |
|
6 |
0.96 |
1.35 |
0.97 |
0.36 |
|
7 |
1.14 |
1.48 |
1.06 |
0.44 |
|
8 |
1.34 |
1.55 |
1.15 |
0.47 |
|
9 |
1.64 |
1.62 |
1.28 |
0.48 |
|
10 |
2.04 |
1.80 |
1.42 |
0.56 |
|
11 |
2.44 |
2.15 |
1.56 |
0.73 |
|
12 |
2.84 |
2.30 |
1.68 |
0.77 |
|
13 |
3.24 |
2.50 |
1.8 |
0.83 |
|
14 |
3.64 |
2.85 |
1.90 |
0.97 |
Table 2: Variation of ƞ/ƞo with concentration of 2-propanol at temperature 298K.
|
S. No. |
Concentration (mol dm-3) |
ƞ/ƞ0 |
√C |
(ƞ/ƞ0-1)/√C |
|
1 |
0.16 |
1.06 |
0.40 |
0.15 |
|
2 |
0.32 |
1.14 |
0.56 |
0.25 |
|
3 |
0.48 |
1.22 |
0.69 |
0.31 |
|
4 |
0.64 |
1.27 |
0.80 |
0.33 |
|
5 |
0.80 |
1.31 |
0.89 |
0.34 |
|
6 |
0.96 |
1.39 |
0.97 |
0.40 |
|
7 |
1.14 |
1.52 |
1.06 |
0.49 |
|
8 |
1.34 |
1.61 |
1.15 |
0.53 |
|
9 |
1.64 |
1.76 |
1.28 |
0.59 |
|
10 |
2.04 |
1.92 |
1.42 |
0.64 |
|
11 |
2.44 |
2.22 |
1.56 |
0.78 |
|
12 |
2.84 |
2.35 |
1.68 |
0.80 |
|
13 |
3.24 |
2.57 |
1.8 |
0.87 |
|
14 |
3.64 |
2.75 |
1.90 |
0.92 |
The values of ƞ/ƞ0 obtained were utilized for the determination of intermolecular interactions in aqueous solutions with the help of Jone–Dole equation 1.
(ƞ/ƞo-1)/√C = A+B√C (1)
The value of A and B were obtained from the intercept and slope of linear plots by (ƞ/ƞ0-1)/√C versus √C. The plots obtained for 1-propanol and 2-propanol are given in Figure 1 and 2 respectively.
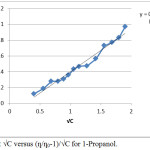 |
Figure 1: Plot √C versus (ƞ/ƞ0-1)/√C for 1-Propanol. |
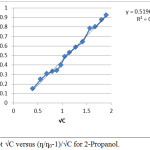 |
Figure 2: Plot √C versus (ƞ/ƞ0-1)/√C for 2-Propanol.Click here to view figure |
The value of A which indicates the ion-ion interaction is almost zero with negative value for both the alcohols. Such negative value of A has been obtained in non- ionic aqueous system.9 In the study for both the solutes (alcohols) value of ƞ/ƞo at each concentrated are also recorded in Table 1 and 2. The coefficient A is expected to be positive for strong electrolyte and zero for non electrolyte.11 Hence the term A√C in equation 1 can be neglected and reduced form of Jone–Dole equation can be applied for alcohols.
ƞ/ƞo = BC + 1 (2)
A plot ƞ/ƞo versus C should be linear having slope value equal to B- coefficient. In case of non- electrolyte the applicability of equation 2 has been considered.12 The plot obtained between ƞ/ƞo and C values clearly shows that the value of intercept is nearly one.
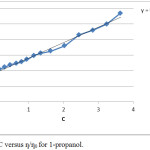 |
Figure 3: Plot C versus ƞ/ƞ0 for 1-propanol. |
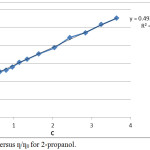 |
Figure 4: Plot C versus ƞ/ƞ0 for 2-propanol.Click here to view figure |
The experimental data for both the alcohols has been utilized to calculate the value of B from the plot ƞ/ƞo versus C. The plots obtained for 1-propanol and 2-propanol are given in Figure 3 and 4 respectively. It is clear from the plots that experimental results in our study justify the applicability of reduced Jone-Dole equation 2. The value of linearity coefficients for both the alcohols is nearly one (0.98). The calculations were carried out with the help of computer and validity of equation was also confirmed graphically. In the analysis the value of slope B is 0.49 mol-1 dm3 for both the alcohols. From the magnitude of B value obtained from Jone-Dole equation is 0.51±0.01 and reduced Jone –Dole equation is 0.49. It can be concluded from these values both the equations have similar results. All the observations clearly suggest that for non-electrolytes reduced Jone-Dole equation can be used for the measurements of solute-solvent interaction. This is an added support for the structure promoting nature of solute as well as the presence of H-H bond forming capability and amphiphilic nature responsible for interaction between the alcohols and water molecule.
In the present study positive value of B indicates a strong alignment of water molecules with alcohols which reveals the “structure making” nature of 1-propanol and 2-propanol in aqueous solutions. The B- coefficients are also known as measure of the order or disorder introduced in the solvent structure by the solutes which suggest that solute – solvent interactions are present in the dilute aqueous solutions of alcohols. It is suggested that the molecules of alcohol form associating clusters through inter – molecular hydrogen bonding13 due to the presence of hydroxyl group.
Acknowledgements
The authors are thankful to Prof. Raj N. Mehrotra, former professor and Head, Chemistry Department, Jodhpur University for useful help with valuable suggestion.
Conflict of Interest
There is no conflict of interest.
References
- Loshali, R.; Kandpal, N.D. Asian J. Chem. 2017, 29, 2701-2703
- Joshi, B.K.; Kandpal, N.D. Phy. Chem. Liq. 2007, 45, 463
- Joshi, R.; Tamta, K.; Chandra, B.; Kandpal, N.D. Int. J. Appl. Chem. 2017, 13, 611-630
- Sondawale, P.J.; Narwade, M. L. Oriental J. Chem. 1997, 13, 169
- Bag, G. C.; Singh, M.N.; Singh, N.R. J. Indian Chem. Soc. 2000, 77, 146
- Jauhar, S.P.; Sadhu, S. Indian J. Chem. 2000, 39(A), 392
- Bag, G.C.; Singh, M.N.; Singh, N.R. J. Indian Chem. Soc. 2001, 78, 294
- Bhat, J. I.; Sreelatha, T.N. Proc. Natl. Acad. Sci. India. 2003, 73, 419
- Kandpal, N.D.; Joshi, B.K. Physics and Chemistry of Liquids. 2009, 47, 250-258
- Nikam, P.S.; Sanwant, A.D. J. Chem. Eng. Data. 1997, 42, 585
- Joshi, R.; Kandpal, N.D. Der Pharmica Lettre. 2015, 7, 126-133
- Akhtar, Y. Int. J. Sci. Tech. and Soc. 2015, 3, 6-9
- Van, N.; Van, W.H.C.; Richtol, J.H.H. J. Phys. chem. 1967, 71, 1483

This work is licensed under a Creative Commons Attribution 4.0 International License.









