Migration Test of Polylactic Acid (PLA) Packaging that Modified with BHT (Butyl Hydroxy Toluene) and TBHQ (Tert Butyl Hydroxy Quinon) Synthetic Antioxidant in Food Simulant
Hanafi1 , Nurdiani2, Septilina Melati Sirait1, Dhina Aprilia Nurani Widyahapsari*1 and Candra Irawan3
, Nurdiani2, Septilina Melati Sirait1, Dhina Aprilia Nurani Widyahapsari*1 and Candra Irawan3
1Department of Food Industrial Quality Assurance Polytechnic of AKA Bogor, Bogor 16154, Indonesia.
2Department of Industrial Waste Treatment Polytechnic of AKA Bogor, Bogor 16154, Indonesia.
3Department of Chemistry Analysis Polytechnic of AKA Bogor, Bogor 16154, Indonesia.
Corresponding Author E-mail: dhinaaprilia1488@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350207
Article Received on : 01-02-2019
Article Accepted on : 27-03-2019
Article Published : 26 Apr 2019
Plastic as food packaging can cause environmental and health problem. One solution for this problems is by substitute conventional plastic into biodegradable plastic such as poly(lactic acid) (PLA). To improve the physical and chemical properties of PLA, synthetic antioxidant such as Butyl Hydroxyl Toluene (BHT) and Tert Butyl Hydroxyl Quinon (TBHQ) were added into PLA. The analysis was carried out on the PLA-BHT and PLA-TBHQ packaging and also food simulants before and after storage at different temperature for 10 days. The analysis included visual appearance, antioxidant activity, total phenol and overall migration. Both PLA-BHT and PLA-TBHQ packaging that were incubated at temperature 40°C had larger migration than at 29°C. The antioxidant activity and total phenol of PLA-TBHQ was higher than PLA-BHT and both of them had decreased antioxidant activity and total phenol during storage but the decreased rate at temperature 40°C was higher than at temperature 29°C.
KEYWORDS:Antioxidant Activity; BHT Antioxidant; Overall Migration; Poly(Lactic Acid) Packaging; TBHQ Antioxidant; Total Phenol
Download this article as:| Copy the following to cite this article: Hanafi H, Nurdiani N, Sirait S. M, Widyahapsari D. A. N, Irawan C. Migration Test of Polylactic Acid (PLA) Packaging that Modified with BHT (Butyl Hydroxy Toluene) and TBHQ (Tert Butyl Hydroxy Quinon) Synthetic Antioxidant in Food Simulant. Orient J Chem 2019;35(2). |
| Copy the following to cite this URL: Hanafi H, Nurdiani N, Sirait S. M, Widyahapsari D. A. N, Irawan C. Migration Test of Polylactic Acid (PLA) Packaging that Modified with BHT (Butyl Hydroxy Toluene) and TBHQ (Tert Butyl Hydroxy Quinon) Synthetic Antioxidant in Food Simulant. Orient J Chem 2019;35(2). Available from: https://bit.ly/2VulwSq |
Introduction
Packaging has an important role at food supply chain because it can maintain food quality and protect food from damages and contamination, as container that facilitates distribution and as communication tool to the consumers.1 Most of plastic packaging are made from synthetic polymers such as petroleum which is known as non renewable and non degradable material. Biodegradable plastics are one of alternative solution to the environmental problem of solid waste form non-biodegradable plastics packaging.2 Biodegradable plastics such as Poly(lactic acid) (PLA), poly(ɛ-caprolactone) (PCL), poly(ethylene glycol) (PEG) and polypropylene glycol (PPG) are noteworthy materials is this respect.3
Polyric lactic acid (PLA) is a biopolymer (polyester) which can be produced from renewable natural resources such as starch and cellulose through lactic acid fermentation.4 PLA can be used as an alternative to conventional polymers because it has biodegradable properties and comes from renewable resources.5 To improve the chemical properties of packaging such as plastics and styrofoam can be added with some materials that are used as dyes, antioxidants, ultraviolet absorber, heat stabilizers, viscosity reducers, acid absorber, peroxide decomposers, lubricants, plasticizers and others.6 The antioxidant substances can avoid or minimized effect of oxidation. There are two types of antioxidants, synthetic and natural. Natural antioxidants are more expensive and less effective than synthetic antioxidants. Synthetic antioxidants that are permitted in food such as BHT (Butyl Hydroxy Toluene), TBHQ (Tert Butyl Hydroxy Quinon), BHA (Butyl Hydroxy Anisol) and others.7
Based on the food packaging terminology, transfer of substance originating from packaging material to its content is called migration. The substance that is migrated can be monomers, solvents, additives such as antioxidant. There are two types of migration: overall migration and specific migration. Overall migration is the total migration period from packaging to food or food simulants under certain conditions. While specific migrations are identifiable substances that migrate from packaging into food or food simulants. The migration process (displacement) into foodstuff may cause dangerous effect to human health and decreases the food quality.8 Based on that study, the objective of this research is to know the migration process of PLA material that is modified with synthetic antioxidant substances (BHT and TBHQ) into food simulants during storage at different temperatures. This research may give suggestion about effect of synthetic antioxidant modified PLA as food packaging.
Materials and Method
The materials used in this research are Polylactic Acid (PLA) ore, synthetic antioxidant (Butyl Hydroxy Toluene) and (Tert Butyl Hydroxy Quinon), montmorillonite (MMT), Polyethylene Glycol (PEG) 400, DPPH (1,1-diphenyl-2-picryl- hydrazyl), chloroform, 3% acetic acid, 15% ethanol, water, Folin-Ciocalteau reagents, gallic acid standard, anhydrous sodium carbonate, FeCl3, and Potassium Bromide.
Instrumentations
The tools used are: ceramic container, magnetic stirrer, stainless steel wire, vortex-Corning® LSETM 120V, porcelain plate, analytical balance-AND GR-200, centrifuge, hotplate, oven, waterbath, visible light spectrophotometre, UV-Visible spectophotometre, erlenmeyer, bakker glass, vial, reaction tube, aluminum foil, and other glassware.
Procedures
PLA Packaging Production
Poly(lactic acid) was modified with synthetic antioxidants Butyl Hydroxy Toluene (BHT) and Tert Butyl Hydroxy Quinon (TBHQ) by heating method at 55°C. PLA ore 5 g was added with 100 ml chloroform, heated at 55°C and 750 rpm for 45 minute. That solution sequentially added with 0.31 ml PEG, 0.25 mg montmorillonite, 0.5 g TBHQ or BHT antioxidant and heated at 55°C for 15 minute in every addition step. Visual appearance, antioxidant activity and total phenol content were, then, analyzed. Antioxidant activity was analyzed using radical scavenger method by Elmastas9 and total phenol content was analyzed using folin method by Gheldof and Engeseth.10
Production of Food Simulant
Migration of PLA-TBHQ and PLA-BHT substance into food simulant were analyzed. Type of food simulant can be seen in Table 1.
Table 1: Food Simulant Type.
| Food Type | Food Simulant |
| Watery food/low level alcohol food | Water |
| High level alcohol food | 15% Ethanol |
| Acidic food | 3% Acetic acid |
Overall Migration Test
Migration test of modified poly(lactic acid) into food simulants after storage at temperature 29°C and 40°C for 10 days using method by Simoneau11 that was modified. PLA membrane was cut into 5 cm x 2.5 cm and it was weighted. Samples specimen were putted at supporting wire and soaked into food simulants solution then incubated at 29°C and 40°C for 10 days. Furthermore, PLA membrane and supporting wire were washed with food simulants solution. Food simulants solution were putted at cup porcelain that the weight was known before. The samples were evaporated using waterbath at 30°C for alcoholic samples and 80°C for non-alcoholic sample then dried at 105°C for 2 hour. Samples residue in cup porcelain was weighted. Beside samples, blank was analyzed too. Overall migration of was determined by the mass of residue after evaporation of food simulant using this equation:
![]()
M: overall migration.
ma : mass residue of sample after evaporation (mg).
mb : mass residue of blank (mg).
S : area (dm2).
Result and Discussion
Visual Appearance of PLA-BHT and PLA-TBHQ Membrane
Visual appearances of PLA membranes that were modified with synthetic antioxidant BHT and TBHQ shown in Fig 1. Based on that result, PLA membrane that was modified with BHT had white transparent color and rough texture. While PLA membrane that was modified with TBHQ had smoother texture than PLA membrane that was modified with BHT. It is because TBHQ has white crystal form and solve in non polar solution meanwhile BHT solve in hydrocarbon solution.
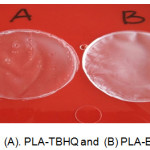 |
Figure 1: (A). PLA-TBHQ and (B) PLA-BHT. |
Antioxidant Activity of PLA-BHT and PLA-TBHQ Membrane
IC50 values indicate antioxidant activity strength, in which antioxidant activity is very strong if IC50 value is below 50 ppm, strong activity if IC50 value is 50 – 100 ppm, moderate activity if IC50 value ranges from 101 to 150 ppm, and weak activity if IC50 value is above 500 ppm.12 Antioxidant activity of PLA that was modified with BHT and TBHQ antioxidant before and after stored at 29°C and 40°C for 10 days shown in Fig 2 and Fig 3. It can be seen that the antioxidant activities of BHT and TBHQ are equally classified as weak antioxidant activity because the antioxidants are not in a pure state, but already interact with PLA packaging materials. TBHQ had stronger antioxidant activity than BHT. BHT had weak antioxidant activity because BHT must be mixed with BHA to have better activity.13
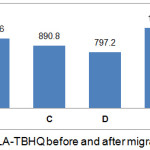 |
Figure 2: Antioxidant activity of PLA-TBHQ before and after migration into food simulant. |
A : PLA-TBHQ before migration; B : PLA-TBHQ with ethanol food simulant at 29°C; C : PLA-TBHQ with acetic acid food simulant at 29°C; D : PLA-TBHQ with water food simulant at 29°C; E : PLA-TBHQ with ethanol food simulant at 40°C; F : PLA-TBHQ with acetic acid food simulant at 40°C; G : PLA-TBHQ with water food simulant at 40°C.
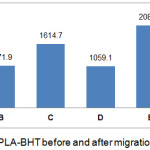 |
Figure 3: Antioxidant activity of PLA-BHT before and after migration into food simulant. |
A : PLA-TBHQ before migration; B : PLA-TBHQ with ethanol food simulant at 29°C; C : PLA-TBHQ with acetic acid food simulant at 29°C; D : PLA-TBHQ with water food simulant at 29°C; E : PLA-TBHQ with ethanol food simulant at 40°C; F : PLA-TBHQ with acetic acid food simulant at 40°C; G : PLA-TBHQ with water food simulant at 40°C
After storage period, PLA-TBHQ and PLA-BHT that were used to package any food simulants had increased of IC50 value (Fig 2 and Fig 3). It means the antioxidant activity was significantly decreased during stored for 10 days at 29°C and 40°C, except that was used to package water food simulant and stored at 29°C. Antioxidant will migrate into food simulant solution and reached equilibrium at 29°C while migration rate increase at 40°C.14
According to Fig 2 and Fig 3, antioxidant activity of PLA-BHT was higher in decreasing than PLA-TBHQ because TBHQ has 2 hydroxyl group meanwhile BHT only has 1 hydroxyl group. Higher hydroxyl group means higher antioxidant activity. Iniguez15 said that hydroxyl group quantity will influence the migration rate and interaction between antioxidant and PLA polymer. BHT synthetic antioxidant only has 1 hydroxyl group so that it has high diffusivity during migration into food simulant at temperature 40°C.
Total Phenol Test of PLA-BHT and PLA-TBHQ Membrane
According to Fig 4 and Fig 5, it is generally known that the total phenol in the modified PLA pack has decreased after interacting with the food simulant for 10 days. It can be caused by the temperature and interactions between PLA-BHT and PLA-TBHQ with food simulators over 10 days. The BHT antioxidant had only slightly decreased of total phenol compare to TBHQ antioxidant. This can be caused by the nature of BHT which is more difficult to oxidize. According to Winarno,16 phenolic compound damage can be caused by the reaction of phenolic compounds of the quinol form transformed into quinone compounds. This reaction is influenced by oxidation reaction factors that occur around food. In addition, the effect of migration by food simulants can be seen from the nature of antioxidant type solubility, namely BHT antioxidants which is more difficult to be soluble in fat, ethanol and acetic acid so that the migration process on ethanol and acetic acid simulants becomes more difficult.
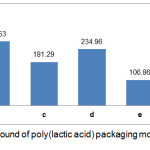 |
Figure 4: Total phenolic compound of poly(lactic acid) packaging modified with antioxidant BHT before and after migration. |
A : PLA-BHT before migration; b : PLA-BHT with ethanol food simulant at 29°C; c : PLA-BHT with acetic acid food simulant at 29°C; d : PLA-BHT with water food simulant at 29°C; e : PLA-BHT with ethanol food simulant at 40°C; f : PLA-BHT with acetic acid food simulant at 40°C; g : PLA-BHT with water food simulant at 40°C.
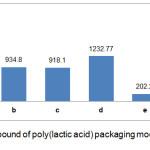 |
Figure 5: Total phenolic compound of poly(lactic acid) packaging modified with antioxidant TBHQ before and after migration. |
A : PLA-TBHQ before migration; b : PLA-TBHQ with ethanol food simulant at 29°C; c : PLA-TBHQ with acetic acid food simulant at 29°C; d : PLA-TBHQ with water food simulant at 29°C; e : PLA-TBHQ with ethanol food simulant at 40°C; f : PLA-TBHQ with acetic acid food simulant at 40°C; g : PLA-TBHQ with water food simulant at 40°C.
Overall Migration Test of PLA-BHT and PLA-TBHQ Packaging Into Food Simulant
According to Table 2, overall migration both of PLA-TBHQ and PLA-BHT packaging material into food simulant that was stored at temperature 29°C was lower than at temperature 40°C. Based on postulate Van’s Hoff Rule, every 10°C temperature increase, the diffusion rate of PLA material into food or food simulants will increase twofold. Diffusion rate will increase interaction between food simulant and modified PLA.6
Table 2: Total migration of modified PLA using antioxidant into food simulant.
|
Type Antioxidant |
Interaction Temperature |
Food Simulant |
Total Migration (mg/dm2) |
| Tert Butyl Hydroxy Quinon (TBHQ) | 29°C (room temperature) | Water |
33.6 |
| 15% ethanol |
55.2 |
||
| 3% acetic acid |
3.2 |
||
| 40°C | Water |
231.2 |
|
| 15% ethanol |
763.2 |
||
| 3% acetic acid |
250.8 |
||
| Butyl Hydroxy Toluene (BHT) | 29°C (room temperature) | Water |
110.0 |
| 15% ethanol |
38.0 |
||
| 3% acetic acid |
9.2 |
||
| 40°C | Water |
297.6 |
|
| 15% ethanol |
919.1 |
||
| 3% acetic acid |
467.2 |
Total migration of 15% ethanol and 3% acetic acid at temperature 40°C were higher than water food simulant. This result was appropriate with visual appearance of ethanol and acetic acid that can be dissolved PLA especially at temperature 40°C so the residue that was produced by ethanol and acetic acid food simulant during overall migration test was higher. Beldi17 reported that migration of some antioxidant were effected by increasing fat content and increasing temperature.
PLA-BHT was easy to migrate into any kind of food simulant at temperature 29°C and 40°C compared to PLA-TBHQ (Table 2). Antioxidant migration character of BHT and TBHQ were caused by hydroxyl group. Iniguez15 said that hydroxyl group quantity will influence the migration rate and interaction between antioxidant and PLA polymer. BHT synthetic antioxidant only has 1 hydroxyl group so that it has high diffusivity during migration into food simulant.
Conclusion
Temperature and food simulant greatly affect the migration of PLA modified BHT and TBHQ antioxidant packs. PLA-BHT and PLA-TBHQ packaging migrated were larger at temperature 40°C than 29°C. Total migration of PLA-BHT into any different kind of food simulant was larger than PLA-TBHQ. This is related to the nature of BHT solubility in the type of food simulant.
Acknowledgements
This research was funded by Polytechnic of AKA Bogor, Ministry of Industry of Indonesia.
Conflict of Interest
There is no conflict of interest.
References
- Robertson, L.G. CRC Press, New York. 2010.
- Rhim, J.W.; Hong, S.I.; Ha, C.S. LWT Food Science and Technology. 2009, 42(2), 612-617.
- Tanoue, S.; Hasook, A.; Iemoto, Y.; Unryu, T. Polym.Compos. 2006, 27, 256–263.
- Sin, L.T.; Rahmat, R.A and Rahman, W.A. Elevesier. 2012.
- Tang, X and Alavi, S. J. Agric. Food Chem. 2012, 60, 1954–1962.
- Sumarjono, B.S. Portal Media Ilmiah Bidang Kimia dan Kemasan. 2016, 3(1), 139-144.
- Winarsi, H. Kanisius, Yogyakarta. 2007.
- Selke, S.E. M.; Culter, J. D and Hernandez, R. J. Hanser Publications Inc. 2004.
- Elmastas, M.; Gulcin, I.; Isildak, Q.; Einen, A. Journal of The Iranian Chemical Society. 2006, 3(3), 258-266.
- Gheldof, N and Engeseth, N.J. Journal of Agricultural and Food Chemistry. 2002, 50(10), 3050-3055.
- Simoneau, C. European Communities, Luxembourg. 2009.
- Ionita, P. Chem.Pap. 2015, 59(1), 11-16.
- Race, S. Tigmorbooks, United Kingdom. 2009.
- Mascheroni, E.; Guillard, V.; Nalin, F.; Mora, L.; Piergiovanni, L. Journal of Food Engineering. 2010, 98(3), 294-301.
- Iniguez-Franco, F.; Soto-Valdez, H.; Peralta, E.; Ayala-Zavala, J. F.; Auras, R.; Gamez-Meza, N. Journal of Agricultural and Food Chemistry. (2012), 60(26), 6515-6523.
- Winarno, F.G. Gramedia, Jakarta. 1989.
- Beldi, G.; Pastorelli, S.; Franchini, F.; Simoneau, C. Food Additives and Contaminant : Part A. 2012, 29(5), 836–845.

This work is licensed under a Creative Commons Attribution 4.0 International License.









