Kinetics and Thermodynamic Studies of Cu(II) Ion Adsorption Onto Synthetic Zeolite Synthesized from Coal Fly Ash: Effect of the Co-Ions to the Total Adsorption
Ahmad Zakaria1, Witri Djasmasari2, Henny Rochaeni3, Yustinus Purwamargapratala4 and Supriyono*3
1Department of Industrial Waste Treatment, Politeknik AKA Bogor, Bogor 16154, Indonesia.
2Departement of Food Industrial Quality Assurance, Politeknik AKA Bogor, Bogor 16154, Indonesia.
3Department of Chemical Analysis, Politeknik AKA Bogor, Bogor 16154, Indonesia.
4National Nuclear Energy Agency, Kawasan Puspiptek, Serpong 15314, Indonesia.
Corresponding Author E-mail: supriyono272@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350227
Article Received on : 25-01-2019
Article Accepted on : 29-03-2019
Article Published : 24 Apr 2019
Study of kinetics and thermodynamic of Cu(II) ion adsorption onto synthetic zeolite made of coal fly ash have been investigated. The aim of this research was to define the kinetics model and thermodynamic parameters such as Gibbs free energy (DG°), entropy (DS°), and enthalpy (DH°) of adsorption process of Cu(II) ion by synthetic zeolite made of coal fly ash. The effect of the presence of coexisting ion to the efficiency of Cu(II)adsorption had also been investigated. The experimental conditions were 5, 15, 30, 45, 60, 75, 90 minutes for the contact times and 27, 32, 37, 42°C for the temperature. The kinetics data were evaluated using a first-order and a pseudo second-order Lagergren equation. The results revealed that the kinetics data had good correlation with the pseudo second-order kinetics model. Thermodynamic studies indicated that the adsorption process was spontaneous with the increase in entropy and decrease in Gibbs energy. The coexisting Pb(II) or Mn(II) ions decreased the Cu(II) ion adsorption onto synthetic zeolite, but increased the total adsorption capacities.
KEYWORDS:Coal Fly Ash; Co-Ions; Gibbs Free Energy; Kinetics Model; Synthetic Zeolite
Download this article as:| Copy the following to cite this article: Zakaria A, Djasmasari W, Rochaeni H, Purwamargapratala Y, Supriyono S. Kinetics and Thermodynamic Studies of Cu(II) Ion Adsorption Onto Synthetic Zeolite Synthesized from Coal Fly Ash: Effect of the Co-Ions to the Total Adsorption. Orient J Chem 2019;35(2). |
| Copy the following to cite this URL: Zakaria A, Djasmasari W, Rochaeni H, Purwamargapratala Y, Supriyono S. Kinetics and Thermodynamic Studies of Cu(II) Ion Adsorption Onto Synthetic Zeolite Synthesized from Coal Fly Ash: Effect of the Co-Ions to the Total Adsorption. Orient J Chem 2019;35(2). Available from: https://bit.ly/2IDGzMo |
Introduction
Industrial development in various countries led to increase industrial pollution significantly. Hence they growing problem of industrial waste. Heavy metal such as copper is one of the contaminants that have the potential to destroy the system of human physiology and other biological systems when over tolerance level. Metals such as copper are produced by industrial metal plating, alloy, steel, dyes, electrical wiring, insecticides, pipelines, and paint.1 The presence of Cu ions in industrial waste is usually together with other heavy metal ions. In the plating industrial wastes, Cu(II) ion is the fifth largest concentration after metals Fe, Cr, Sn, and Zn, and followed by the metal ions with smaller concentrations, namely Ni, Mn, Pb, Cd, and Ag.2 Some methods for treating heavy metal ions in industrial effluents have been reported in the previous studies.1,3-8 These methods are neutralization, precipitation, ion exchange, biosorption and adsorption. For low metal ion concentration, the adsorption process is the recommended method for taking the metal ion. The process of adsorption involves intermolecular attractive forces, ion exchange, and chemical bonding. Synthetic zeolite derived from coal fly ash is one of the materials that can be used to adsorb heavy metal ions. Reaction of synthetic zeolite is similar to the condition of the earth’s crust. The synthesis is done by making the reactants into a gel and then placed in an autoclave at temperature range 70°C – 150°C.5
The aim of this research was to define the kinetics model and thermodynamic parameters such as Gibbs free energy, entropy and enthalpy of the adsorption process of Cu(II) ion by synthetic zeolite made of coal fly ash and to investigate the effect of the coexisting Pb(II) or Mn(II) ions to the efficiency of Cu(II) ion adsorption.
Materials and Method
The materials are synthetic zeolite made of coal fly ash, while the chemicals are NaOH, HCl, H2SO4, CuSO4, MnCl2, Pb(NO3)2, (all materials are from Merck). The tools used in this experiment are atomic absorption spectrophotometer, water bath with temperature control, oven, shaker, sieve shaker of 40 mesh, pH meter, analytical balance with 0,1 mg accuracy, what man filter paper 42, pipette, 50 ml volumetric pipette, funnel, 50 ml and 100 ml glass flasks and other tools.
Determination of Reaction Order (Kinetics Model)
This experiment was performed after experiments optimization. The adsorbate optimum pH was 4 and adsorbent concentration was 50 mg /100 mL. The experiments were performed by varying the reaction time as the independent variable and the other two as dependent variables. 50 mL of adsorbate solution with a concentration of 80 mg/L (pH optimum) is added to the synthetic zeolite (optimum weight) in 100 mL erlenmeyer glass. Erlenmeyer glass then agitated with a shaker at 150 rpm for a 5, 15, 30, 45, 60, 75, and 90 minutes. Each variation of time, the sample was filtered and the filtrate was measured using the AAS to determine the concentration of Cu (II) ion in solution. From these experiments, it is known that reaction order matches the adsorption system by looking at the value of the determination coefficient on each order of reaction.
Determination of Thermodynamic Parameters
Thermodynamic parameters are determined by varying the temperature of the experiment ; 27, 32, 37 and 42°C with the other variables are constant. This experiment was performed by using 50 mL of adsorbate solution (pH optimum) with a concentration of 80 mg/L. This solution was added into 100 mL erlenmeyer glass that already containing synthetic zeolite (optimum weights) and then agitated for the optimum time. After the sample filtered, and then the filtrate was measured using the AAS to determine the concentration of Cu(II) ion in solution.
Adsorption Experiment for Binary Metal Systems
This experiment was performed by binary adsorption system, in example by adding a number of metal ions Mn or Pb adsorbate into the solution of Cu (II). So it can be known relationship between adsorption capacity of Cu (II) with the presence of Mn or Pb ions and the strength of the interaction of Mn2+ and Pb2+ on the adsorbent. Experiments was done by making an adsorbate solution containing 80 mg/L Cu (II), the combined concentration of 80 mg/L Cu (II) with 25 mg/L Pb(II), and the combined concentration of 80 mg/L Cu (II) with 25 mg/L Mn (II). Then the erlenmeyer was shaked during the optimum time, then the sample was filtered and the filtrate was measured using the AAS.
Calculation
The amount of heavy metal adsorbed by coal fly ash was calculated using the following equation:

Co is the initial concentration (mg L-1), Ce is the final concentration in equilibrium (mg L-1), Qe is adsorption capacity or the concentration of adsorbate on the adsorbent at equilibrium (mg g-1), m is adsorbent mass and V is volume of the adsorbate.
Results and Discussion
Effect of Contact Times
Adsorption kinetics describes the solute retrieval rate by the adsorbent during the adsorption time. This parameter is important because it determines the efficiency of the adsorption process. Effect of contact times on the adsorption capacity can be seen in Fig. 1. In the Fig.1, it can be seen that the adsorption capacity of the adsorbent was increased with the increasing contact time and in 75 minute equilibrium was occurred. The time needed for equilibrium depends on the types and the interaction of the adsorbate and the adsorbent.
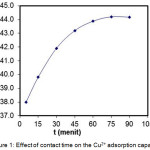 |
Figure 1: Effect of contact time on the Cu2+ adsorption capacity. |
At the beginning of the contact times, the adsorption capacity was increased, but after almost all the active sides interacted with metal ions, the adsorption rate was decreased. So there was no significant increase in the adsorption capacity after the active sides was saturated, so the adsorption rate was only dependent on the migration of metal ions in the liquid phase to the surface of the complex adsorbent-adsorbate.9
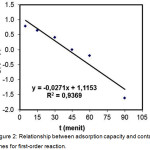 |
Figure 2: Relationship between adsorption capacity and contact times for first-order reaction. |
The first-order and pseudo second-order reaction models were done by plotting t versus log (qe-qt) (Fig. 2) and t versus t/qt as Lagergren equation10 (Figure 3), so that the adsorption rate constant (k), the optimum adsorption capacity (qe) and coefficient determination can be determined.
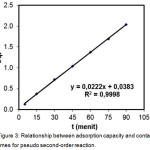 |
Figure 3: Relationship between adsorption capacity and contact times for pseudo second-order reaction. |
Determination coefficient for the first-order reaction was smaller than the pseudo second-order reaction, predictive value of adsorption capacity was compared to the experimental value of the optimum adsorption capacity had an error -70.5% (Table 1). So that the first-order reaction was less suitable to be applied as a model for the adsorption kinetics of synthetic zeolite adsorbent. Therefore, pseudo-second-order reaction with the determination coefficient (R2) > 0.99 (figure 3 and Table 1) was more suitable for the adsorption kinetics of synthetic zeolite adsorbent, with the error of 1,91%. It can be suggested that the kinetic parameters of adsorbent satisfied pseudo second-order reaction because it had a high degree of accuracy in predicting the optimum adsorption capacity.
Table 1: Comparison of first-order, the pseudo second-order rate constants, predictions and experimental qe values.
|
Co (mg/L) |
qe (mg/g) experiment |
First-order reaction |
Pseudo second-order reaction |
||||||
|
K1 (min-1) |
qe (cal) |
% error |
R2 |
K2 (g/mg min) |
qe (cal) |
% error |
R2 |
||
|
80 |
44.2 |
0.062 |
13.04 |
-70.5 |
0.9369 |
0.0130 |
45.04 |
1.91 |
0.9998 |
Effect of Temperature
Adsorption of Cu(II) ions increased with increasing temperature of 300-315° K. (Fig. 4). The increase in the adsorption capacity due to at the higher temperatures enhanced the active surface of the adsorbent, increased metal ion kinetic energy, and decreased the formation of metal ions due to the reduction of the effects of hydration, so that it may penetrate the deeper layers of pores.4
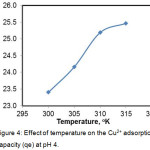 |
Figure 4: Effect of temperature on the Cu2+ adsorption capacity (qe) at pH 4. |
Enthalpy energy ( H°) of the synthetic zeolite adsorbent and Cu(II) adsorbate with concentration of 80 mg/L was 62 KJ mol-1 (Table 2), endothermic. Some studies have been reported by Fan et al. (2008)4 for the adsorbent (Penicillium simplicissium) and adsorbate Cd (II), Zn (II) and Pb (II). In Table 2, the change in entropy of adsorption energies are positive values. From these data it can be concluded that an increase in the degree of irregularity in the adsorbent-adsorbate system, so the metal ions adsorbed on the adsorbent were more disordered.11 This phenomenon in the adsorption system is very beneficial because it can increase the stability of the adsorbent-adsorbate complex.
Table 2: Thermodynamic parameters of Cu(II) adsorption by synthetic zeolite.
| Thermodynamic parameters | |||||
| Adsorbent | Adsorbate Cu2+ (mg L-1) | Temperature (°C) | ΔG° (KJ mol-1) | ΔH° (KJ mol-1) | ΔS° (J mol-1) |
| 27 | -2.2 | 62 | 214 | ||
| Synthetic zeolite | 80 | 32 | -3.27 | ||
| 37 | -4.34 | ||||
| 42 | -5.41 | ||||
The value of the Gibbs free energy of adsorption systems is negative in all experimental temperature conditions (Table 2). This proves that the process was spontaneous adsorption system. The calculation of the free energy at temperature of 27, 32, 37 and 42, the value of which tends to be negative, indicated that the spontaneous adsorption process at higher temperatures.
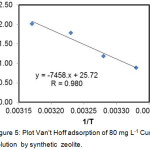 |
Figure 5: Plot Van’t Hoff adsorption of 80 mg L-1 Cu(II) solution by synthetic zeolite. |
Values of thermodynamic parameters of Cu(II) adsorption by synthetic zeolite was obtained from the calculation of the slope, intercept of linear equations and Van’t Hoff plot (Fig.5).
Effect of Co-ions
Mn and Pb heavy metal ions were metal ions that was often found in industrial effluents together with Cu metal ion. In this experiment, adsorbate Cu(II) is made from sulfate salt and applied to a binary system consisting of two types of ions in adsorbate solution, ie metal ions Cu2+ with Mn2+ and Cu2+ with Pb2+. Efficiency of adsorption capacity of Cu2+ ions was influenced by Mn2+ and Pb2+ ions. The presence of these ions (Mn2+, Pb2+) in Cu2+ adsorbate can decrease the efficiency and capacity of the Cu2+ adsorption. (Table 3). This is due to the competition between the metal ions of Cu, Mn and Pb in getting the adsorbent active site to adsorbent-adsorbate complexes.
Table 3: Effect of co-ions on the efficiency of Cu2 + adsorption by synthetic zeolite.
| Adsorbent | Initial concentration (mg L-1) | Adsorption capacity (mg g-1) | Adsorption efficiency (%) | |||||||
| Cu | Pb | Mn | Cu | Pb | Mn | Total | Cu | Pb | Mn | |
| Synthetic zeolite | 80 | – | – | 25.78 | – | – | 25.78 | 96.97 | – | – |
| 80 | 25 | – | 25.4 | 8.3 | – | 33.7 | 95.64 | 100 | – | |
| 80 | – | 25 | 25.16 | – | 3.081 | 28.24 | 94.56 | – | 38.06 | |
The presence of Pb or Mn ions in Cu(II) solution can simultaneously reduce the adsorption capacity of Cu (II), but can improve overall adsorption capacity. This phenomenon is due to a shift in the equilibrium towards the formation of adsorbent-adsorbate complex with increasing concentration of the adsorbate.
Conclusion
Adsorption kinetics was determined as the pseudo second-order. Adsorption reactions tend to be spontaneous and endothermic. The presence of Mn or Pb ions decreases the efficiency of adsorption of Cu2+ but increase the total adsorption capacity.
Acknowledgements
The authors wish to thank to Polilteknik AKA Bogor, Ministry of Industry of Indonesia for providing the research funding. Partial support from National Nuclear Energy Agency Indonesia are also acknowledged.
Conflict of Interest
There is no conflicts of interest.
References
- Sarkar B, Xi Y, Megharaj M, Krishnamurti GSR, Rajarathnam D, Naidu R. Journal of Hazardous Materials. 2010, 183, 87-97.
- Ventkatiswaran P, Vellaichanny S, Palanivelu K. International Journal Environ Sci. Tech. 2007, 4(4), 497-504.
- Gupta SS, Bhattacharayya GK. Journal of Enviromental Management. 2008, 87, 46-58.
- Fan T, Liu Y, Feng B, Zeng G, Yang C, Zhou M, Zhou H, Tan Z, Wang X. Journal of Hazardous Materials B. 2008, 160, 655-661.
- Chang-Han Lee, Matthew S.A. Journal of Env. Sci. Int. 2013. 22(10), 1327-1335.
- Lita D, Suprihanto N, Enri D. Journal Eng. Technol. Sci. 2017, 49(4), 546-559.
- Lei X, Xuebo Z, Hongbiao C, Zhenqiu Z, Jiani L, Jing Z. Bioinorganic Chemistry and Application. 2017, 3695604.
- Ajay K.A, Mahendra S.K, Chandrashekhar P.P, Ishwardas L.M. Journal of Chem. Technol. And Metallurgy. 2015, 50(5), 601-605.
- Yu B, Zhang Y, Shukla A, Shukla SS, Dorris KL. Journal of Hazardous Materials B. 2000, 80, 33-42.
- Talal S. Chem. Eng. Research and Design. 2015, 96, 172-176.
- Kubilay RS, Gurkan A, Savran T, Sahan. Adsorption. 2007, 13, 41-51.
- Hui KS, Chao CYH, Kot SC. Journal of Hazardous Materials. 2005, 127, 89-101.

This work is licensed under a Creative Commons Attribution 4.0 International License.









