Formation of Copper Powders in the Cathode Half-Period of Alternating Current
Abduali Bayeshov1, Azhar Bayeshova2, Dinara Abizhanova1 and Umida Abduvaliyeva*1
1Institute of Fuel, Electrochemistry and Catalysis named after D.V. Sokolskiy.
2Kazakh National University named after al-Farabi, Almaty, Kazakhstan.
Corresponding Author E-mail: abdumida14@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350225
Article Received on : 12-10-2018
Article Accepted on : 13-03-2019
Article Published : 17 Apr 2019
In the proposed research paper, we considered the pattern of formation of copper powders during the polarization of electrodes by transient currents. When a copper-titanium pair of electrodes is polarized with an industrial alternating current of 50 Hz in a solution of copper (II) sulfate, it is shown that copper powders with a high current yield are formed on the surface of the titanium electrode. Under optimum conditions, the current yield is 80%. The size of the formed copper powders is 0.5-2 µm. The analysis of the obtained oscillograms showed that when titanium is used in the circuit, the asymmetrical sinusoidal current flows.
KEYWORDS:Alternating Current; Copper; Powder; Titanium
Download this article as:| Copy the following to cite this article: Bayeshov A, Bayeshova A, Abizhanova D, Abduvaliyeva U. Formation of Copper Powders in the Cathode Half-Period of Alternating Current. Orient J Chem 2019;35(2). |
| Copy the following to cite this URL: Bayeshov A, Bayeshova A, Abizhanova D, Abduvaliyeva U. Formation of Copper Powders in the Cathode Half-Period of Alternating Current. Orient J Chem 2019;35(2). Available from: https://bit.ly/2IqbPyh |
Introduction
There are several methods for obtaining copper powders. The main one is the mechanical physico-chemical method. Usually, metal powders of large and different forms are obtained by mechanical means. This includes cutting, drilling, grinding, and other methods. Electrochemical cementation, thermal spraying of dry salts, and autoclave methods can be attributed to the physico-chemical methods. However, among these methods, the electrochemical method holds a special place, and today over 90% of copper powders are obtained by this method. Until today, the most dispersed copper powders (30-80 μm) are obtained by this method.1-10
Copper powders obtained by electrochemical methods are used in metallurgy, chemistry, electrical engineering, and other fields. In electrical engineering, they are widely used in the manufacture of copper-graphite contacts, in chemical production as a catalyst, and in hydrometallurgy as a cementing agent.
It has been established that ultra-disperse copper powders in the oil composition reduces the mechanical friction, and wear of iron parts. This phenomenon is widely used in developed countries.11
The specificity of the reaction in the electrodes is investigated in detail when obtaining copper powders. Copper powder is formed at the cathodes. The study of the reduction of copper (II) ions forming copper powders at the cathode and the effect on this process of other electrochemical parameters of the composition of electrolyte presented is in our earlier research works.12-14
Our research works have shown that when copper powders are produced by compound of a sulfuric acid of copper (II) solution with tetravalent titanium ions, the dispersion of metal powders obtained at the cathode increases, and also significantly increases the current consumption.
In all known works, copper powders are obtained using the direct current. In such a case, it is necessary to use expensive current settings.
Our preliminary studies have shown that in a certain case, metallic powders can be obtained through polarization of the electrodes in an alternating current with a frequency of 50 Hz of sulfuric acid in a copper (II) solution. Therefore, in the submitted research papers, we examined the pattern of formation of copper powders when the electrode is polarized with transient current.
Work Procedure
The electrolysis was carried out in a 100 mL glass beaker. Copper in the form of a plate and titanium in the form of a wire were used as electrodes. The purity of copper was 99.99%, and the elemental purity of titanium was 97.6%. Preliminary studies have shown that the formation of copper powder depends on the current density at the titanium electrode and on the concentration of copper(II) ions in the solution. In this works, the effect on current density on titanium electrodes was in the range of 0-125 kA m-2, and the impact of copper(II) ions was in the range of 0-30 g L-1. The electrolysis time was 30 minutes. Copper powders formed after the electrolysis, were washed with distilled water, and to prevent oxidation, they were rinsed with 0.05% sodium soapy water. The current consumption of the formed copper powders was calculated for the cathodic half-period of alternating current. The current was measured using an amperometer assigned for alternating current. The laboratory transformer was used to adjust the strength of the alternating current. A schematic diagram of the electrolysis device is shown in Fig. 1. During the study, an oscillogram of transient currents passing through the electrochemical circuit is recorded.
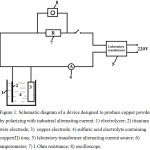 |
Figure 1: Schematic diagram of a device designed to produce copper powder by polarizing with industrial alternating current: 1) electrolyzer; 2) titanium wire electrode; 3) copper electrode; 4) sulfuric acid electrolyte containing copper(II) ions; 5) laboratory transformer alternating current source; 6) amperometer; 7) 1 Ohm resistance; 8) oscilloscope. |
Results and Discussion
During the polarization of a copper-titanium pair of electrodes with alternating current, copper powders are formed on the surface of titanium. The effect the current density in titanium electrodes varies from 0 to 125,000 A m-2 (125 kA m-2) on the size of copper powders formed is determined. As it can be seen from Fig. 2, an increase in the current density at the titanium reaching 75 kA m-2 as the current consumption (CC) of copper powders formed at this electrode increases. A further increase in the current density leads to a decrease in the CC of copper powder formation reaching 53.5% at 125 kA m-2.
![Figure 2: Impact of titanium electrode current density on the current consumption of the formation of copper powders, during polarization of copper-titanium electrodes pair with alternating current: [Cu2+] = 20 g L-1, [H2SO4] = 100 g L-1, τ = 30 minutes.](http://www.orientjchem.org/wp-content/uploads/2019/04/Vol35No2_For_Abd_fig2-150x150.jpg) |
Figure 2: Impact of titanium electrode current density on the current consumption of the formation of copper powders, during polarization of copper-titanium electrodes pair with alternating current: [Cu2+] = 20 g L-1, [H2SO4] = 100 g L-1, τ = 30 minutes. |
The formation of copper powders during placing the copper-titanium pair of electrodes into a solution of sulfuric acid with copper(II) ions can be explained with follows:
With an increase in current density in titanium electrodes, a metal oxide is formed with a semiconducting “valve property” on the surface of titanium electrodes at the initial moment of the anode half-period of alternating current, and the current flow slows down in this direction, and in the cathode half-period of a transient current of the titanium electrode the electric current passes through this electrode unimpeded. At that moment, the current becomes higher than the current density, which is limited within the electrode, and the copper (II) ions forming the copper powders are oxidized, and the crystals of the copper powders formed in the anodic half-period can be melted in a certain amount. As a result, round-shaped copper powders are formed. The reduction in the current consumption of the formation of copper powders at a high current density can be explained by the additional reaction that occurs at this moment – the exhale of hydrogen gas.
The effect of concentration of copper(II) ions in the range of 0-30 g L-1 was studied (Fig. 3). The results of the study show that an increase in the concentration of copper (II) ions leads to an increase in the current consumption for the formation of copper powders. It is established that when the concentration of copper(II) ions is 30 g L-1, the current consumption is 80%.
![Figure 3: The effect of the concentration of copper (II) sulfate on the current consumption and the formation of copper powders: [H2SO4] = 100 g L-1, CC = 75 kA -2, τ = 30 minutes.](http://www.orientjchem.org/wp-content/uploads/2019/04/Vol35No2_For_Abd_fig3-150x150.jpg) |
Figure 3: The effect of the concentration of copper (II) sulfate on the current consumption and the formation of copper powders: [H2SO4] = 100 g L-1, CC = 75 kA -2, τ = 30 minutes. |
When the concentration of sulfuric acid is zero, due to a sharp increase in pH at the cathode surface in the initial moments of electrolysis, copper hydroxide is formed and in the range of 50-100 g L-1the concentration does not affect properly the electrolysis process, and at a still ongoing higher concentration current consumption, the formation of copper powders is reduced. This phenomenon can be explained by the dissolution of the formed metal powders due to an increase in the acidity of the solution.
Fig. 4 shows an oscillogram during the polarization of copper-titanium pair of electrodes with industrial alternating current. During the polarization of two graphite electrodes with 50 Hz transient current (Fig. 4(a)) we can see the passage of the correct sinusoidal current through the electrochemical circuit. When using a copper-titanium pair of electrodes, there is an amount of current at the anode half-period of alternating current (Fig. 4(b)). When copper(II) ions are added to the solution in the oscillogram, minor changes occur (Fig. 4(c)).
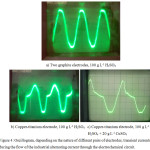 |
Figure 4: Oscillogram, depending on the nature of different pairs of electrodes, transient currents during the flow of the industrial alternating current through the electrochemical circuit. |
The shapes and sizes of copper powders obtained at different current densities at the titanium electrodes are photographed with an electron microscope and evaluated (Figure 5). In all cases, the formation of roundish copper powders with a size of about 0.5-2.0 μm has been established.
![Figure 5: Microphotograph of copper powders obtained during polarization with alternating: a) iTi = 125 kA m-2, [Cu(II)] = 20 g L-1, [H2SO4] = 100 g L-1; b) iTi = 75 kA m-2, [Cu(II)] = 20 g L-1, [H2SO4] = 100 g L-1; c) iTi = 75 kA m-2, [Cu(II)] = 30 g L-1, [H2SO4] = 100 g L-1.](http://www.orientjchem.org/wp-content/uploads/2019/04/Vol35No2_For_Abd_fig5-150x150.jpg) |
Figure 5: Microphotograph of copper powders obtained during polarization with alternating: a) iTi = 125 kA m-2, [Cu(II)] = 20 g L-1, [H2SO4] = 100 g L-1; b) iTi = 75 kA m-2, [Cu(II)] = 20 g L-1, [H2SO4] = 100 g L-1; c) iTi = 75 kA m-2, [Cu(II)] = 30 g L-1, [H2SO4] = 100 g L-1. |
In conclusion, it is shown that during polarization of a copper-titanium pair of electrodes in sulfuric acid/copper(II) solution by industrial alternating current with frequency of 50 Hz, copper powder is formed on titanium electrode surface with a high current consumption. It has been established that the current density in titanium electrodes and the concentration of copper(II) ions in solution do not significantly affect the current consumption. In the optimal case (iTi= 75 kA m-2, [Cu(II)] – 30 g L-1) the current consumption is 80%. It has been established that during polarization with an alternating current, round-shaped copper powders with a size of about 0.5-2 μm are formed. The results of the study showed that it is possible to obtain ultra-disperse copper powder by electrochemical means, without using industrial current in special devices.
Acknowledgements
The work was supported by the Ministry of Education and Science of the Republic of Kazakhstan (АР05131096).
Conflict of Interest
There is no conflict of interest.
References
- Sansan Yu; Shuangming Li; Xin Ge; Mingju Niu; Hao Zhang; Can Xu; and Wenxiu Li. Influence of Reducing Atmosphere of Subcritical/Supercritical Mild Alcohols on the Synthesis of Copper Powder. Industrial & Engineering Chemistry Research, 2014, 53 (6), 2238-2243.
- Nakazawa Gen-ichi. Study on the Precipitation of Copper Powder from Aqueous Cupric Sulphate Solution by Hydrogen Pressure Reduction. Journal of the Society of Materials Science, Japan, 1965, 14, 725-729.
- Ashour Owais. Effect of electrolyte characteristics on electrowinning of copper powder. J. Appl. Electrochem. 2009, 39, 1587–1595.
- Yepifantseva, T. A.; Fedorov, D. M.; Kayuk, V. G.; Shtern, M. B.; Martyukhin, I.D. Production of copper powder from rolled scale and its mechanical properties. Powder Metallurgy and Metal Ceramics, November 2010, 49, 366–369.
- Navneet Singh Randhawa; Deepak Chandra Sau; Manoj Kumar. Direct electrolytic refining of end-of-life industrial copper waste scraps for production of high purity copper powder. Russian Journal of Non-Ferrous Metals, July 2016, 57, 367–373.
- Pavlov, Ye. A.; Udalova, T.A.; Grigoreva, T.F.; Lyakhov, N.Z. Preparing Ultradisperse Copper Powder via the Mechanochemical Reduction of Copper Oxides by Magnesium. Bulletin of the Russian Academy of Sciences: Physics, May 2018, 82, 574–577.
- Juanjuan Wang; Xiaolian Chao; Guangzhao Li; Lajun Feng; Kang Zhao. Fabrication and enhanced characterization of copper powder filled copper calcium titanate/poly(vinylidene difluoride) composite. Journal of Materials Science: Materials in Electronics, April 2017, 28, 5435–5439.
- Sha Zhang; Yungui Li; Rong Wang; Zhonghui Xu; Bin Wang; Shu Chen; Mengjun Chen. Superfine copper powders recycled from concentrated metal scraps of waste printed circuit boards by slurry electrolysis. Journal of Cleaner Production, 20 May 2017, 152, 1-6.
- Yingying Chu; Mengjun Chen; Shu Chen; Bin Wang; Kaibin Fu; Haiyan Chen. Micro-copper powders recovered from waste printed circuit boards by electrolysis. Hydrometallurgy, July 2015, 156, 152-157.
- Sha Zhang; Yungui Li; Rong Wang; Zhonghui Xu; Bin Wang; Shu Chen; Mengjun Chen. Superfine copper powders recycled from concentrated metal scraps of waste printed circuit boards by slurry electrolysis. Journal of Cleaner Production, 20 May 2017, 152, 1-6.
- Songping Wu. Preparation of fine copper powder using ascorbic acid as reducing agent and its application in MLCC. Materials Letters, February 2007, 1125-1129.
- Bayeshov, A.B. Electrochemical methods of extraction of copper, chalcogenes and synthesis of their compounds, Nauka Kaz SSR, 1990, 108.
- Bayeshov, A.B.; Bayeshova, A.K.; Bayeshova, S.A. Electrochemistry, Kazakh University, Almaty, 2014, 316.
- Bayeshov, A.B.; Kozhakov, B.Ye., Buketov, Ye.A. The method of copper powder producing. А.С. USSR No. 1082066, July 17, 1982. (Not subject to publication in public media).

This work is licensed under a Creative Commons Attribution 4.0 International License.









